Alkaline Electrocatalysis
The interest for alkaline fuel cells (AFC), linked to the possibility to use non-carbon fuels in substitute to hydrogen and non-Pt electrocatalysts without performance losses, has been renewed in the last decade, owing to the development of performant alkaline electrolyte membranes. We address the topic of AFC by focusing on three aspects: (1) materials and mechanisms of electrooxidation of non-carbon fuels of the boron and nitrogen families (for direct AFC), (2) non-Pt electrocatalysts for the oxygen reduction reaction (ORR) and (3) durability of AFC electrocatalysts.
(1) Materials and mechanisms of electrooxidation of non-carbon fuels of the boron and nitrogen families (NaBH4, N2H4, N2H4BH3, NH3BH3)
We study the mechanisms of complex fuel oxidation (e.g. BH4- oxidation reaction, BOR) on base metals (Pt, Au, Ag, Pd, Ni) using a combination of physicochemical (FTIR spectroscopy 1-3, differential electrochemical mass spectrometry: DEMS (Figure 1) 4-6) and electrochemical techniques (rotating ring-disk electrode (Figure 2) 5-7, electrochemical impedance spectroscopy (Figure 3) 8, 9) at planar and tailored nanostructured electrodes, to understand the elementary steps of the reaction (Figure 4).
The interest for alkaline fuel cells (AFC), linked to the possibility to use non-carbon fuels in substitute to hydrogen and non-Pt electrocatalysts without performance losses, has been renewed in the last decade, owing to the development of performant alkaline electrolyte membranes. We address the topic of AFC by focusing on three aspects: (1) materials and mechanisms of electrooxidation of non-carbon fuels of the boron and nitrogen families (for direct AFC), (2) non-Pt electrocatalysts for the oxygen reduction reaction (ORR) and (3) durability of AFC electrocatalysts.
(1) Materials and mechanisms of electrooxidation of non-carbon fuels of the boron and nitrogen families (NaBH4, N2H4, N2H4BH3, NH3BH3)
We study the mechanisms of complex fuel oxidation (e.g. BH4- oxidation reaction, BOR) on base metals (Pt, Au, Ag, Pd, Ni) using a combination of physicochemical (FTIR spectroscopy 1-3, differential electrochemical mass spectrometry: DEMS (Figure 1) 4-6) and electrochemical techniques (rotating ring-disk electrode (Figure 2) 5-7, electrochemical impedance spectroscopy (Figure 3) 8, 9) at planar and tailored nanostructured electrodes, to understand the elementary steps of the reaction (Figure 4).
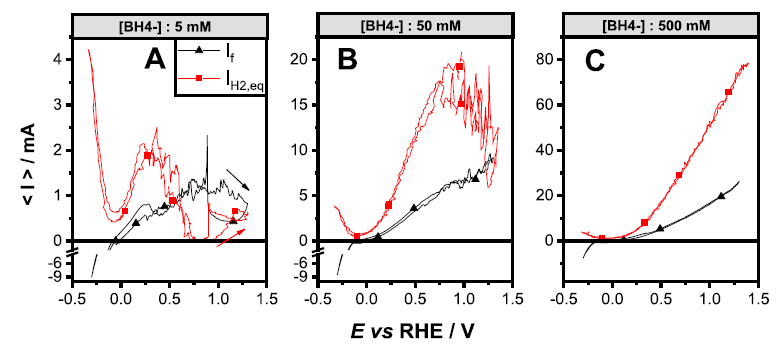
Figure 1: faradaic current (black) and hydrogen evolution current (red) measured for a Pd electrode during the BOR in various experimental conditions.
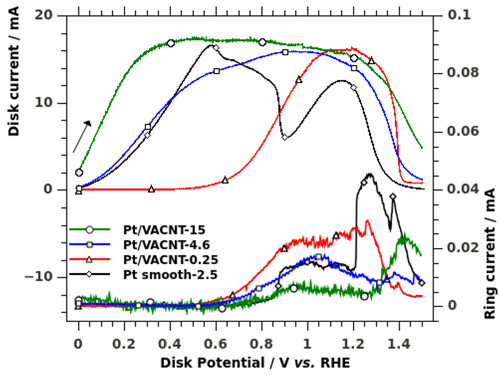
Figure 2: RDE voltammograms of 10 mM NaBH4 in 1 M NaOH at 25 mV s−1 and 2500 rpm (positive-going scan). The disk is composed of the different Pt surfaces; the Au-ring is held at +0.2 V vs. RHE to detect BH3OH- intermediates. Reproduced from reference 7 with permission from Elsevier.
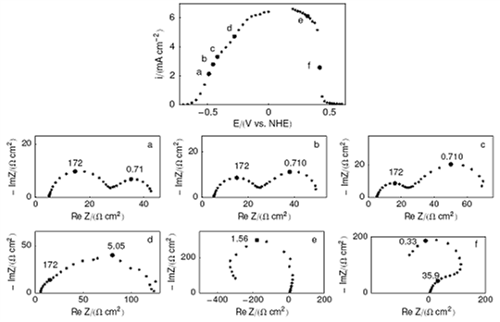
Figure 3: (j vs. E) plot and six Nyquist diagrams of the gold electrode impedance obtained at various potentials (a, b, c, d in the region of increasing currents, e, f in the region of the gold deactivation) at 400 rpm. Graphs parametered in Hz. Reproduced from 8 with permission from Elsevier.
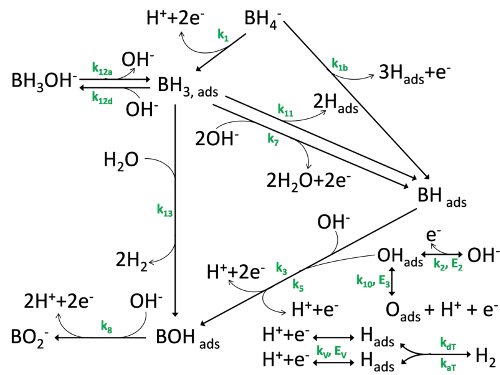
Figure 4: BOR mechanism proposed following the results of the physicochemical and electrochemical techniques detailed above.
This strategy is presently deployed for other fuels of the boron and nitrogen families (NH3BH3, N2H4, N2H4 BH3) 4, 10, in collaboration with the Universidade de São Paulo (Fabio H. B. Lima, Edson A. Ticianelli, Brazil), the Université de Montpellier (Umit Demirci, France) and the University of California (Plamen Atanassov, Irvine, USA). It shall enable to tailor performant electrocatalysts for practical direct AFC (DAFC), and has led to prepare highly-efficient Ni-based (and PGM-free electrocatalysts) for the BOR, enabling fast reaction well-below the hydrogen potential (Figure 5) 11.
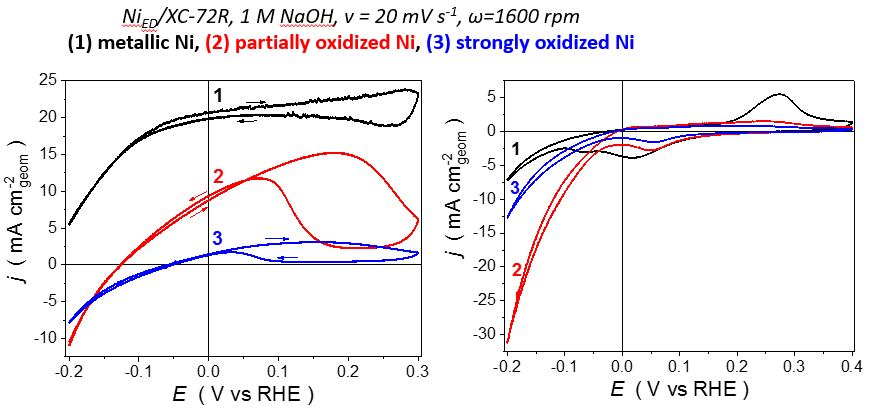
Figure 5: Nickel electrocatalysts for the BOR.
(2) Nanostructured manganese oxides for the alkaline ORR
In complement to the study of the anode electrocatalysts, DAFC require the development of fuel-tolerant electrocatalysts. Nanostructured manganese oxides supported on carbon black, developed in coll. with the Czech Academy of Science (Jiri Vondràk, Czech Republic) 12-14 are materials of choice for that purpose, should it be for borohydride-tolerant ORR electrocatalysts 15, 16 or ethanol-tolerant ones (Figure 5) 17.
In complement to the study of the anode electrocatalysts, DAFC require the development of fuel-tolerant electrocatalysts. Nanostructured manganese oxides supported on carbon black, developed in coll. with the Czech Academy of Science (Jiri Vondràk, Czech Republic) 12-14 are materials of choice for that purpose, should it be for borohydride-tolerant ORR electrocatalysts 15, 16 or ethanol-tolerant ones (Figure 5) 17.
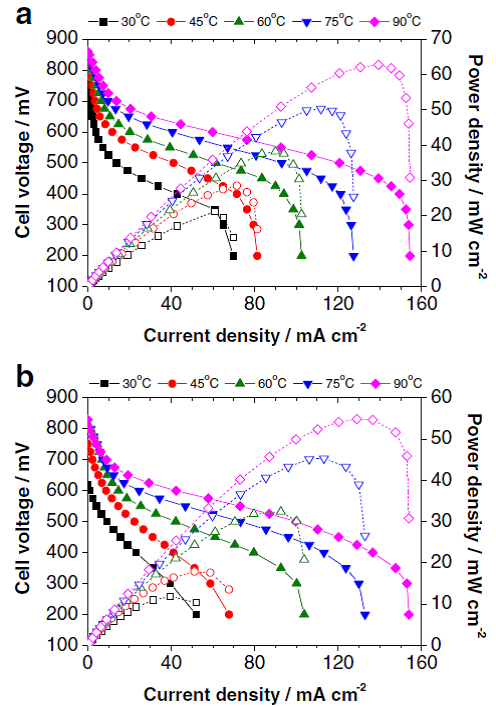
Figure 6: cell potential and Power density versus current densities for alkaline single cells operating at different temperatures for a) Pt/C and b) NiMnOx/C cathode materials. Reproduced from 17 with permission from Springer.
(3) Durability of AFC electrocatalysts
We also evaluate the durability of electrocatalysts for alkaline fuel cells. The results show that PGM/C materials are not stable in alkaline operation, and that their main mechanism of degradation is linked to the detachment of the metal nanoparticles from the carbon support (Figure 7) 18-23.
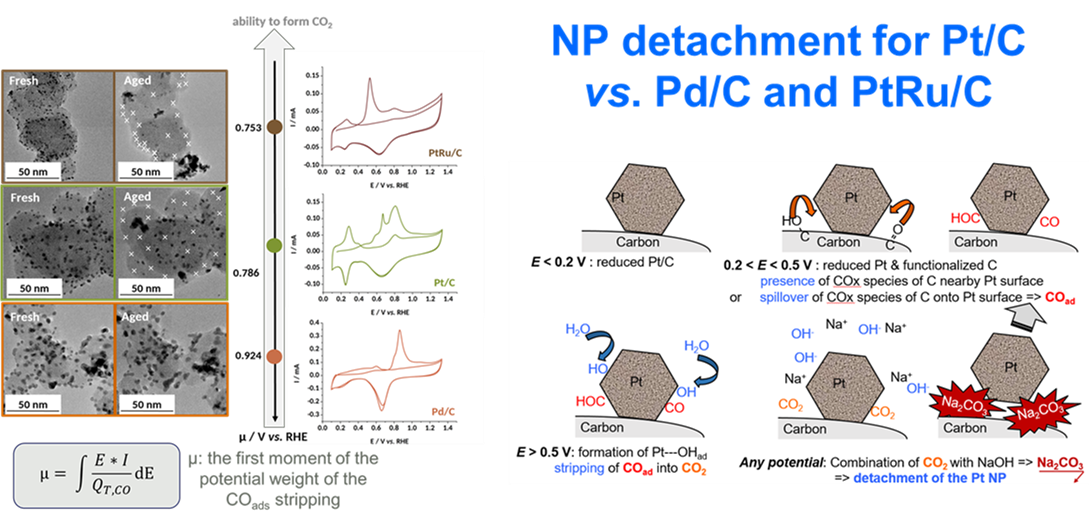
Figure 7: micrographs of several PGM/C electrocatalysts before/after potential cycling in 0.1 M NaOH, 25°C and proposed (main) mechanism of degradation.
We also evaluate the durability of electrocatalysts for alkaline fuel cells. The results show that PGM/C materials are not stable in alkaline operation, and that their main mechanism of degradation is linked to the detachment of the metal nanoparticles from the carbon support (Figure 7) 18-23.

Figure 7: micrographs of several PGM/C electrocatalysts before/after potential cycling in 0.1 M NaOH, 25°C and proposed (main) mechanism of degradation.
Contact : Marian Chatenet
REFERENCES
1- Molina Concha, B.; Chatenet, M.; Coutanceau, C.; Hahn, F. Electrochem. Commun. 2009, 11, (1), 223-226.
2- Molina Concha, B.; Chatenet, M.; Maillard, F.; Ticianelli, E. A.; Lima, F. H. B.; de Lima, R. B. Phys. Chem. Chem. Phys. 2010, 12, (37), 11507-11516.
3- Molina Concha, B.; Chatenet, M.; Ticianelli, E. A.; Lima, F. H. B. J. Phys. Chem. C 2011, 115, (25), 12439-12447.
4- Belén Molina Concha, M.; Chatenet, M.; Lima, F. H. B.; Ticianelli, E. A. Electrochim. Acta 2013, 89, 607-615.
5- Pasqualeti, A. M.; Olu, P.-Y.; Chatenet, M.; Lima, F. H. B. ACS Catal. 2015, 5, (5), 2778-2787.
6- Braesch, G.; Bonnefont, A.; Martin, V.; Savinova, E. R.; Chatenet, M. Electrochim. Acta 2018, 273, 483-494.
7- Olu, P.-Y.; Bonnefont, A.; Rouhet, M.; Bozdech, S.; Job, N.; Chatenet, M.; Savinova, E. Electrochim. Acta 2015, 179, 637-646.
8- Chatenet, M.; Molina-Concha, B.; Diard, J.-P. Electrochim. Acta 2009, 54, 1687-1693.
9- Parrour, G.; Chatenet, M.; Diard, J.-P. Electrochim. Acta 2010, 55, 9113-9124.
10- Zadick, A.; Petit, J.-F.; Martin, V.; Dubau, L.; Demirci, U. B.; Geantet, C.; Chatenet, M. ACS Catal. 2018, 8, 3150-3163.
11- Oshchepkov, A. G.; Braesch, G.; Ould-Amara, S.; Rostamikia, G.; Maranzana, G.; Bonnefont, A.; Papaefthimiou, V.; Janik, M. J.; Chatenet, M.; Savinova, E. R. ACS Catal. 2019, 9, 8520-8528.
12- Vondrak, J.; Klapste, B.; Velicka, J.; Sedlarikova, M.; Reiter, J.; Roche, I.; Chainet, E.; Fauvarque, J. F.; Chatenet, M. J. New Mat. Electrochem. Sys. 2005, 8, (3), 209-2
13- Roche, I.; Chainet, E.; Chatenet, M.; Vondrak, J. J. Phys. Chem. C 2007, 111, (3), 1434-1443.
14- Roche, I.; Chainet, E.; Vondrak, J.; Chatenet, M. J. Appl. Electrochem. 2008, 38, (9), 1195-1201.
15- Chatenet, M.; Micoud, F.; Roche, I.; Chainet, E.; Vondrak, J. Electrochim. Acta 2006, 51, (25), 5452-5458.
16- Garcia, A. C.; Lima, F. H. B.; Ticianelli, E. A.; Chatenet, M. J. Power Sources 2013, 222, (0), 305-312.
17- Garcia, A.; Linares, J.; Chatenet, M.; Ticianelli, E. Electrocatal. 2014, 5, (1), 41-49.
18- Zadick, A.; Dubau, L.; Zalineeva, A.; Coutanceau, C.; Chatenet, M. Electrochem. Commun. 2014, 48, 1-4.
19- Zadick, A.; Dubau, L.; Sergent, N.; Berthomé, G.; Chatenet, M. ACS Catal. 2015, 5, (8), 4819-4824.
20- Zadick, A.; Dubau, L.; Demirci, U. B.; Chatenet, M. J. Electrochem. Soc. 2016, 163, (8), F781-F787.
21- Lafforgue, C.; Chatenet, M.; Dubau, L.; Dekel, D. R. ACS Catal. 2018, 8, 1278-1286.
22- Lafforgue, C.; Zadick, A.; Dubau, L.; Maillard, F.; Chatenet, M. Fuel Cells 2018, 18, (3), 229-238.
23- Lafforgue, C.; Maillard, F.; Martin, V.; Dubau, L.; Chatenet, M. ACS Catal. 2019, 9, (6), 5613−5622.
REFERENCES
1- Molina Concha, B.; Chatenet, M.; Coutanceau, C.; Hahn, F. Electrochem. Commun. 2009, 11, (1), 223-226.
2- Molina Concha, B.; Chatenet, M.; Maillard, F.; Ticianelli, E. A.; Lima, F. H. B.; de Lima, R. B. Phys. Chem. Chem. Phys. 2010, 12, (37), 11507-11516.
3- Molina Concha, B.; Chatenet, M.; Ticianelli, E. A.; Lima, F. H. B. J. Phys. Chem. C 2011, 115, (25), 12439-12447.
4- Belén Molina Concha, M.; Chatenet, M.; Lima, F. H. B.; Ticianelli, E. A. Electrochim. Acta 2013, 89, 607-615.
5- Pasqualeti, A. M.; Olu, P.-Y.; Chatenet, M.; Lima, F. H. B. ACS Catal. 2015, 5, (5), 2778-2787.
6- Braesch, G.; Bonnefont, A.; Martin, V.; Savinova, E. R.; Chatenet, M. Electrochim. Acta 2018, 273, 483-494.
7- Olu, P.-Y.; Bonnefont, A.; Rouhet, M.; Bozdech, S.; Job, N.; Chatenet, M.; Savinova, E. Electrochim. Acta 2015, 179, 637-646.
8- Chatenet, M.; Molina-Concha, B.; Diard, J.-P. Electrochim. Acta 2009, 54, 1687-1693.
9- Parrour, G.; Chatenet, M.; Diard, J.-P. Electrochim. Acta 2010, 55, 9113-9124.
10- Zadick, A.; Petit, J.-F.; Martin, V.; Dubau, L.; Demirci, U. B.; Geantet, C.; Chatenet, M. ACS Catal. 2018, 8, 3150-3163.
11- Oshchepkov, A. G.; Braesch, G.; Ould-Amara, S.; Rostamikia, G.; Maranzana, G.; Bonnefont, A.; Papaefthimiou, V.; Janik, M. J.; Chatenet, M.; Savinova, E. R. ACS Catal. 2019, 9, 8520-8528.
12- Vondrak, J.; Klapste, B.; Velicka, J.; Sedlarikova, M.; Reiter, J.; Roche, I.; Chainet, E.; Fauvarque, J. F.; Chatenet, M. J. New Mat. Electrochem. Sys. 2005, 8, (3), 209-2
13- Roche, I.; Chainet, E.; Chatenet, M.; Vondrak, J. J. Phys. Chem. C 2007, 111, (3), 1434-1443.
14- Roche, I.; Chainet, E.; Vondrak, J.; Chatenet, M. J. Appl. Electrochem. 2008, 38, (9), 1195-1201.
15- Chatenet, M.; Micoud, F.; Roche, I.; Chainet, E.; Vondrak, J. Electrochim. Acta 2006, 51, (25), 5452-5458.
16- Garcia, A. C.; Lima, F. H. B.; Ticianelli, E. A.; Chatenet, M. J. Power Sources 2013, 222, (0), 305-312.
17- Garcia, A.; Linares, J.; Chatenet, M.; Ticianelli, E. Electrocatal. 2014, 5, (1), 41-49.
18- Zadick, A.; Dubau, L.; Zalineeva, A.; Coutanceau, C.; Chatenet, M. Electrochem. Commun. 2014, 48, 1-4.
19- Zadick, A.; Dubau, L.; Sergent, N.; Berthomé, G.; Chatenet, M. ACS Catal. 2015, 5, (8), 4819-4824.
20- Zadick, A.; Dubau, L.; Demirci, U. B.; Chatenet, M. J. Electrochem. Soc. 2016, 163, (8), F781-F787.
21- Lafforgue, C.; Chatenet, M.; Dubau, L.; Dekel, D. R. ACS Catal. 2018, 8, 1278-1286.
22- Lafforgue, C.; Zadick, A.; Dubau, L.; Maillard, F.; Chatenet, M. Fuel Cells 2018, 18, (3), 229-238.
23- Lafforgue, C.; Maillard, F.; Martin, V.; Dubau, L.; Chatenet, M. ACS Catal. 2019, 9, (6), 5613−5622.



