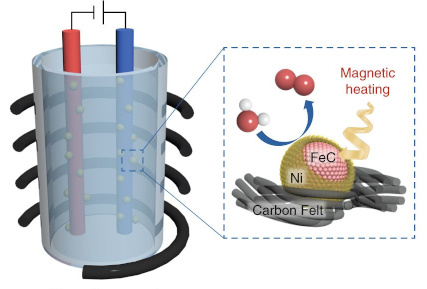
Improved water electrolysis using magnetic heating of FeC–Ni core–shell nanoparticles
L’utilisation de champs magnétiques alternatifs permet d’intensifier la réaction électrochimique de dégagement de l’oxygène.
Par Christiane Niether, Stéphane Faure, Alexis Bordet, Jonathan Deseure, Marian Chatenet, Julian Carrey, Bruno Chaudret & Alain Rouet