Person in charge: Virginie Roche (Pr UGA)
Amorphous metals, high entropy or pseudo high entropy multi-element alloys
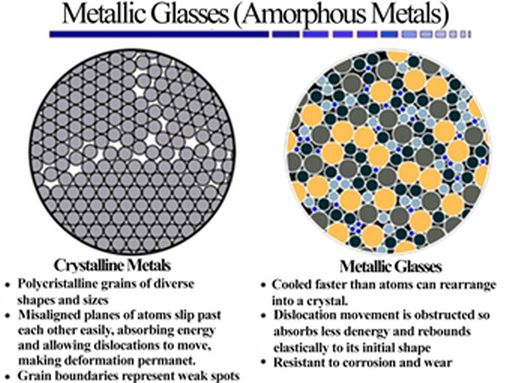
Since 2011, we have been working on characterising the corrosion resistance properties of metallic glass alloys as part of a collaboration with the SIMaP laboratory in Grenoble and the Federal University of São Carlos (UFSCar) in Brazil. A metallic glass is a solid material whose disordered atomic structure is obtained when its liquid state is cooled without crystallisation. It is therefore a solid that has local atomic order (on a small scale), but no large-scale order. In general, metals are among the most difficult materials to vitrify because they tend to crystallise easily during cooling. However, with rigorous control of alloy composition and processing, a wide variety of alloys can now be produced in a glassy state. We are particularly interested in the use of iron-based amorphous metals, often as coatings, which has increased in recent years due to their good corrosion resistance and tribological properties. The high corrosion resistance of these materials is due to their high compositional homogeneity, the absence of crystalline defects (grain boundaries, dislocations) that can be preferential areas for corrosion, and the need for lower passivating element contents. The extremely high corrosion resistance of certain amorphous alloys is considered to be a consequence of the rapid formation of thin, uniform passive films without weak points and with a high concentration of corrosion-resistant alloying elements. The possibility of obtaining amorphous coatings from iron-based alloys using ‘average’ purity processes and elements is interesting because, among other things, it reduces costs.In addition, we are also working on biocompatible Fe- or Mg-based metallic glass materials for their biodegradation properties and good mechanical properties, for applications in the biomedical field as alternative materials (or coatings) to titanium-based alloys.
Magnesium alloys
Influence of the microstructure of Mg alloys on corrosion rate
As part of this work on magnesium alloys for biomedical applications, one of the key points was to slow down the rate of degradation of crystalline Mg in a physiological environment. The addition of zinc appears to be a promising approach, as its positive effects have been shown to be linked both to its ease of shaping for the production of metallic glass and to at least partial passivation of the surface in physiological environments. We therefore focused on two alloy compositions, Mg60Zn34Ca6 and Mg73Zn23Ca4, in physiological solution (SBF). The corrosion properties of the two compositions were studied and compared. This work has been published in the Journal of Alloys and Compounds (doi:10.1016/j.jallcom.2018.09.346), and the detailed results can be found in the article.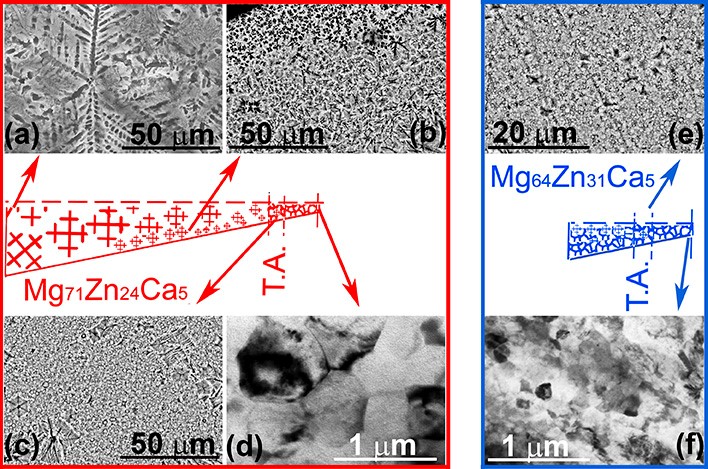
Figure 4 : Microstructures obtained for the two compositions.
The figure above shows the microstructures obtained for the two alloy compositions. A microstructure gradient appeared during cooling after casting, allowing the corrosion properties to be examined for each average microstructure size.
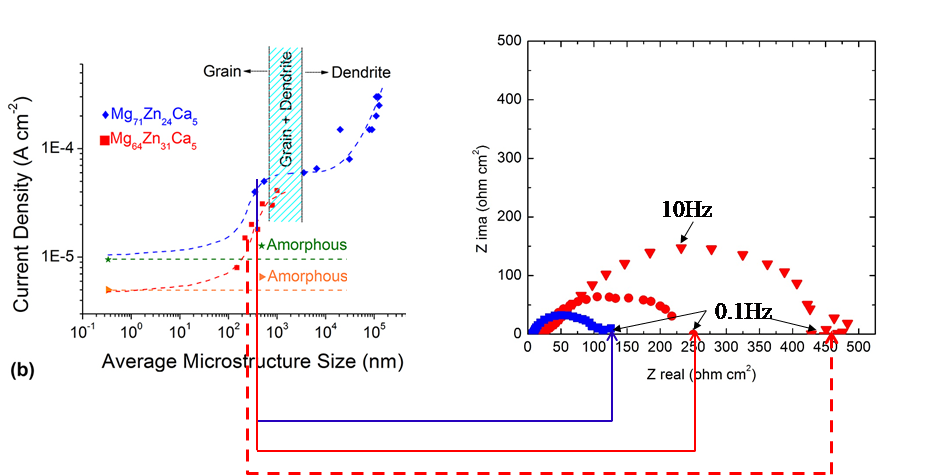
Figure 5 : Evaluation of the corrosion resistance of different microstructures for the two compositions
For a similar average microstructure size, increasing the amount of zinc reduces the corrosion rate, and the more the average microstructure size decreases for a given composition, the more the corrosion rate decreases for the two compositions studied, Mg60Zn34Ca6 and Mg73Zn23Ca4, in physiological solution.
The following figure shows the proposed mechanism:
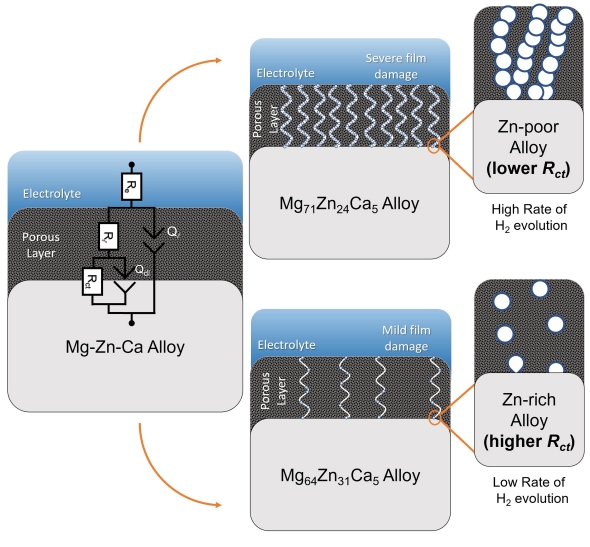
Figure 6 : Proposed mechanism for the dissolution of Mg60Zn34Ca6 and Mg73Zn23Ca4 in physiological solution
Influence of mechanical deformation on the microstructure of magnesium alloys and their corrosion properties
This requires the insertion of reconstruction implants (fracture fixation plates, screws, etc.), which generally need to be removed at a later stage, requiring surgery and carrying a risk of infection. The insertion of bioresorbable bone implants would eliminate this risk. Proper bio-resorption requires the implant to dissolve at a rate such that its degradation, accompanied by a loss of mechanical integrity, is closely correlated with the bone reconstruction process. The kinetics of bone reconstruction observed in the human body vary from 4 to 24 weeks depending on the site of implantation. This physiological variability in bone reconstruction rates naturally affects the kinetics of implant dissolution, which must therefore be able to adapt to these physiological constraints. The other physiological constraint is related to the implant's ability to transmit mechanical stress during the transitional period of bone reconstruction: biocompatible and bioresorbable alloys must also have mechanical properties compatible with the area of the human body where they are implanted. The aim of the project is therefore to generate Mg alloys with a controlled microstructure in order to obtain alloys with corrosion kinetics that can be adjusted according to the physiological constraints of the implantation site. These alloys must also exhibit mechanical properties that evolve during degradation in line with tissue reconstruction kinetics. This work is being carried out in collaboration with Simap et the University of Louvain la Neuve.Mg alloys for stents
Since 2021, we have been using electrochemical techniques to study the dissolution behaviour of coated and uncoated Mg-Nd-Zn-Zr alloy stents. A study in an organic electrolyte has enabled us to investigate the initial stages of dissolution of these stents. The electrochemical impedance results show constant phase element (CPE) behaviour, attributed to the layers on the stent surface, as well as diffusion impedance associated with the diffusion of magnesium ions. A power law model has been proposed to characterise the electrical properties of the layers. In addition, samples with oxide layers exhibited a high-frequency distribution that was well described by complex ohmic impedance. Different types of coatings were evaluated. Compared to bare and plasma-pretreated stents, organic parylene coatings exhibited lower capacitance values, associated with a thicker layer that remains after stent opening. Other organic coatings were evaluated in a synthetic physiological liquid solution.
Surface functionalisation and modelling of the electrochemical impedance of complex surfaces
Another aspect of this research concerns surfaces and interfaces and their interaction with service environments. In-depth knowledge of these interactions is a major challenge in terms of the functionalisation or structuring of new materials. In this context, we are interested, on the one hand, in the formation and structure of TiO2 nanotube layers, which can be considered model surfaces given their strong topological structure (see photo below – Figure 2) and which are developed on pure titanium. We also analyse them using electrochemical impedance spectroscopy in order to cross-reference topological and electrochemical information. We have recently shown that the topological evolution of TiO2 nanotube layers, combined with an alternative representation of impedance diagrams, reveals behaviours that are more complex than those currently reported in the literature, notably highlighting a third time constant that cannot be identified using conventional impedance diagram processing methods.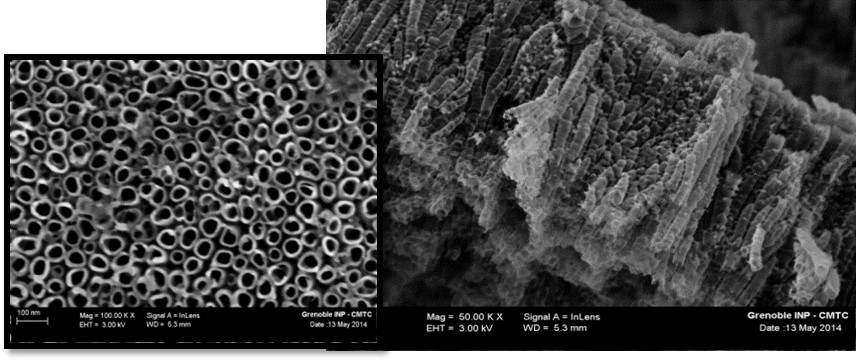
Figure 2 : Top view and cross-section of TiO₂ nanotubes produced by electrochemical means
Corrosion under stress
We work with stress rings (Figure 1) to measure stress corrosion in various applications. These phenomena are difficult to predict and lead to rapid crack propagation, which can significantly alter the mechanical properties of cables and threaten their resistance to stress and their service life. The stresses evaluated will simulate the presence of stress accumulation. Several types of samples can be examined: flat, wire or multi-wire systems.
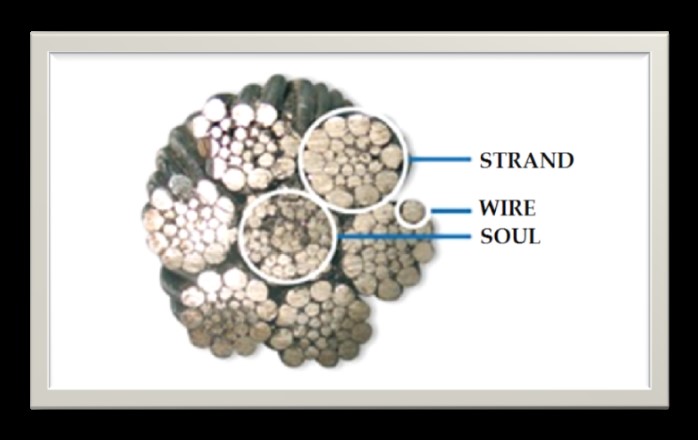

Figure 1 : Picture of a cross-section of a cable used for passenger transport and a tension ring.



