This thematic is dedicated to the phenomena of transport, permeation and charge transfer as well as to the study of electrochemical interfaces for electrochemical conversion and storage. The approach is to couple electrochemical characterization with other physico-chemical techniques and/or modeling. The high temperature activity focuses on the study of mixed conductors used as gas electrodes in fuel cells, electrolysers and oxygen-permeable membranes. The functioning mechanisms are studied by impedance spectroscopy under polarization and cyclic voltammetry. The low temperature activity is dedicated to the analysis of i) ion transport mechanisms in liquid and polymer electrolytes by coupling with impedance spectroscopy and pulsed field gradient NMR, ii) electrochemical mechanisms (conversion mechanism (Li/S), transport process in intercalation materials, dendritic growth on Li metal) and iii) degradation processes of active materials of positive and negative and current collectors.
High temperature solid oxide systems
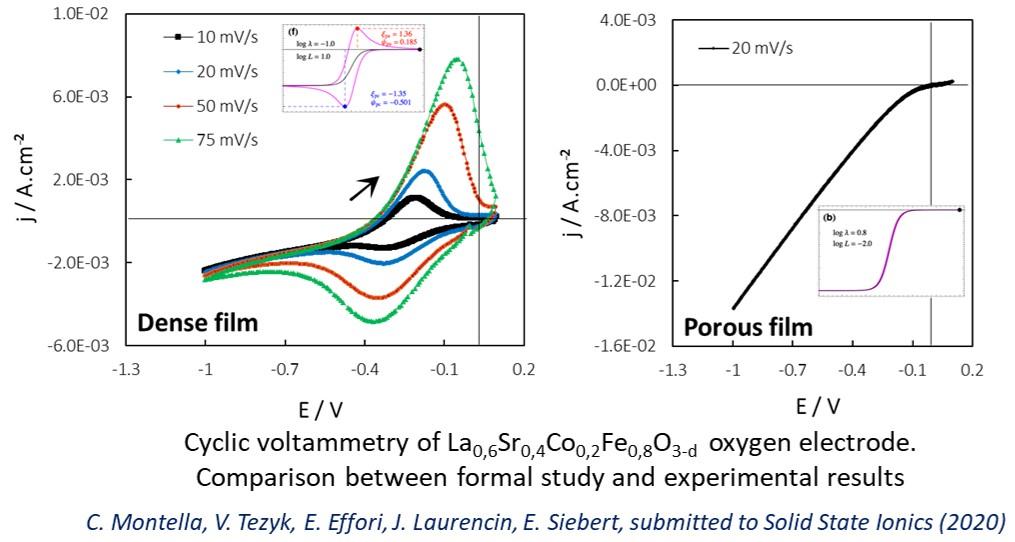
This activity focuses on the development of electrochemical characterizations in half-cell and complete cell to study the reaction mechanisms and understand the degradation processes in SOFCs and SOEC. Modelling of the (i) kinetics of the oxygen electrode reaction and (ii) co-electrolysis H2O and CO2 to HT (collaboration with CEA/LITEN) simulate polarization curves, impedance spectra under polarization of porous electrodes and emitted gases. Cyclic voltammetry was used for the first time to assess the effect of oxygen surface exchange kinetics on the change in stoichiometry of materials. Another component of this activity concerns the development of catalytic anode for SOFC batteries running on carbon-based fuels such as methane (collaboration with IRCELYON) or ethanol (collaboration with Brazil). Studies have also been carried out on the transport properties of mixed conductive materials (electrodes, selective membranes for oxygen and membrane reactors) and complete solid electrolyte cells for the measurement of oxygen activity in extreme media (liquid sodium, nuclear fuels).
Study of Li-metal/electrolyte interfaces and intercalation/electrolyte material
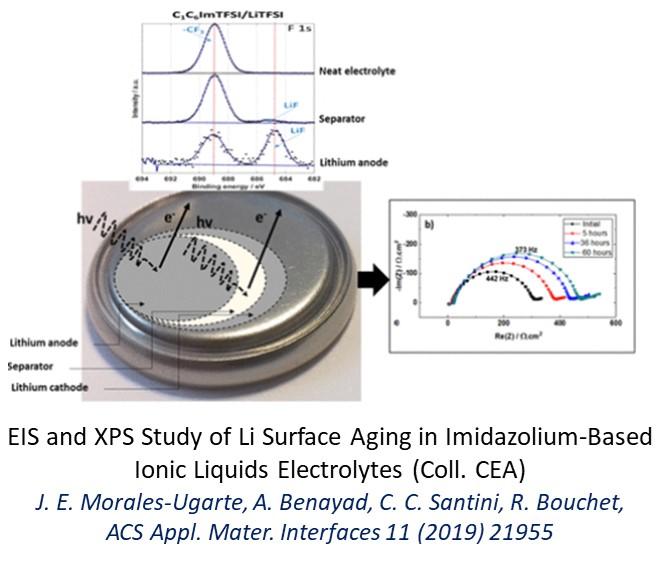
Studies on the analysis of the interfacial reactivity of Li metal have focused on the impact of electrolyte chemistry on the chemical and electrical nature of the Solid Electrolyte Interphase (SEI) that forms on Li. In particular, the objective is to decorrelate the contribution of ion transport (single-ion vs binary conductor), the nature and homogeneity of SEI and the microstructure of Li on the dendritic growth processes of Li. We have thus developed relevant methodologies by coupling impedance spectroscopy and chronopotentiometry / chronoamperometry, but also by relying on tools sensitive to surface chemistry (XPS), or topology (X microtomography). Studies have also been devoted to the study of interfacial kinetics and transport processes in intercalation materials by exploiting thin film models by coupled measurements of GITT and impedance spectroscopy. The development and electrochemical study of fluorinated solvents was also conducted.
Magnesium battery
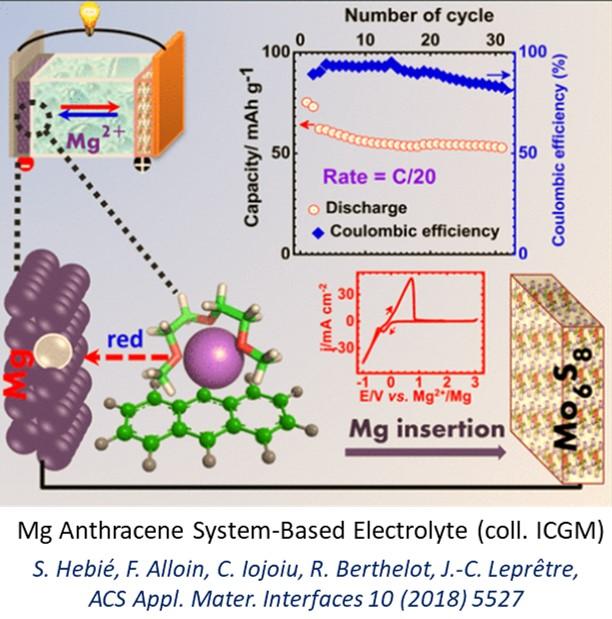
This activity essentially addressed the design of high-performance liquid electrolytes, the control of the Mg metal/electrolyte interface and the synthesis and characterizations of organic positive electrodes. Our studies have led to the development of liquid electrolytes with the required properties using either i) original Mg borohydrides, used as electrolyte salts, or ii) electron-rich molecules π, used as electrolyte additives.