
Carbon-capped nanoparticles for efficient and durable hydrogen oxidation reaction in alkaline fuel cells
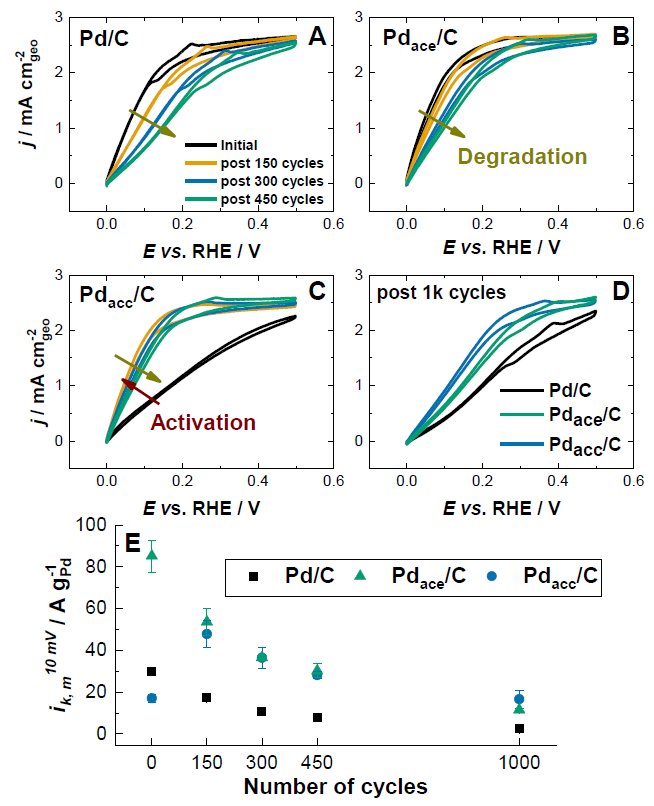
Alkaline fuel cells enable to use non-noble catalysts, owing to the larger activity/stability of most transition metals in alkaline than in acidic environments. While for the oxygen reduction reaction, MnOx 1-7 or Fe-N-C 8 catalysts have proven their competitiveness versus Pt, only Pt yields fast hydrogen oxidation reaction (HOR) in alkaline environments. For that reason, LEPMI-EIP strives to search for Pt/C alternatives for the anode of alkaline fuel cells.Palladium-based catalysts could be an option, but their stability in alkaline environment is not granted (even if it is better than that of Pt/C) 9-12. For that reason, we have chosen to protect the Pd nanoparticles by a carbon cap, that should limit the Pd/C degradation phenomena. We have shown that the carbon capping limits the Pd-based nanoparticles’ agglomeration, detachment, and metal leaching, improving long-term catalyst durability in HOR-like operation. Thicker carbon layers lead to higher materials durability and lower degradation rate (larger performance stability) upon accelerated stress test, compared to thinner carbon layers and of course to unprotected Pd/C (Figure 1). In addition, the carbon-capped catalysts enable to maintain better the required Pd/PdO state of the surface that is essential for fast HOR, resulting in superior intrinsic HOR activity versus unprotected Pd/C (Figure 2) 13.

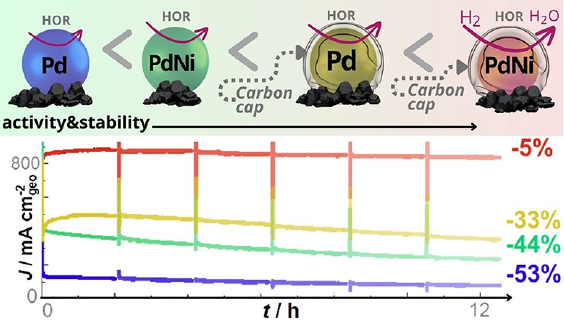
The strategy was fulfilled as well with PdNi alloyed nanoparticles, and tests conducted in anion-exchange membrane fuel cells (coll. ICGM, Université de Montpellier); the results show that the approach is very successful, leading to significantly enhanced HOR activity and durability for the carbon-capped PdNi/C catalyst versus the carbon-capped Pd/C catalyst and the uncapped counterparts (Figure 3) 14.
Oxidation of complex fuels in alkaline environments
An alkaline environment is frequently used to stabilize hydrogen-rich species, that can be used as hydrogen carriers in fuel cells; examples are sodium borohydride (NaBH4), ammonia borane (N3BH3) and hydrazine (N2H4). Of course, the presence of this alkaline stabilizing alkaline solution makes more relevant to use alkaline conditions also for the fuel cell. In addition, it is worth mentioning that harvesting the maximum energy content of these species relies on the direct oxidation of these fuels, more than on their first decomposition into hydrogen and then hydrogen oxidation on a classical anode, because these fuels have significantly lower reversible potential than H2 (e.g. -0.4 V vs RHE for NaBH4 and -0.32 V vs RHE for N2H4). This is why the EIP team of LEPMI strives to valorise/oxidize these fuels directly, in the most complete manner possible. The complexity of the reactions at stake (many electrons/hydroxyle ions involved) makes necessary to unveil the reaction mechanisms, so that ultimately the electrocatalysts and electrode composition/structure can be optimized.Studying such complex fuels oxidation mechanisms is done by using complementary electrochemical and physicochemical analyses. While in the past this has been done for noble metal surfaces for NaBH4 15-17 and boranes 17-19, it is worth mentioning that such metals are not stable when nanodispersed on a carbon support, as is the case in real fuel cell electrodes 10-12,20,21. This is why the team recently turned its attention to more available/less costly metals, like nickel, which of course consists of a more sustainable and desirable approach.
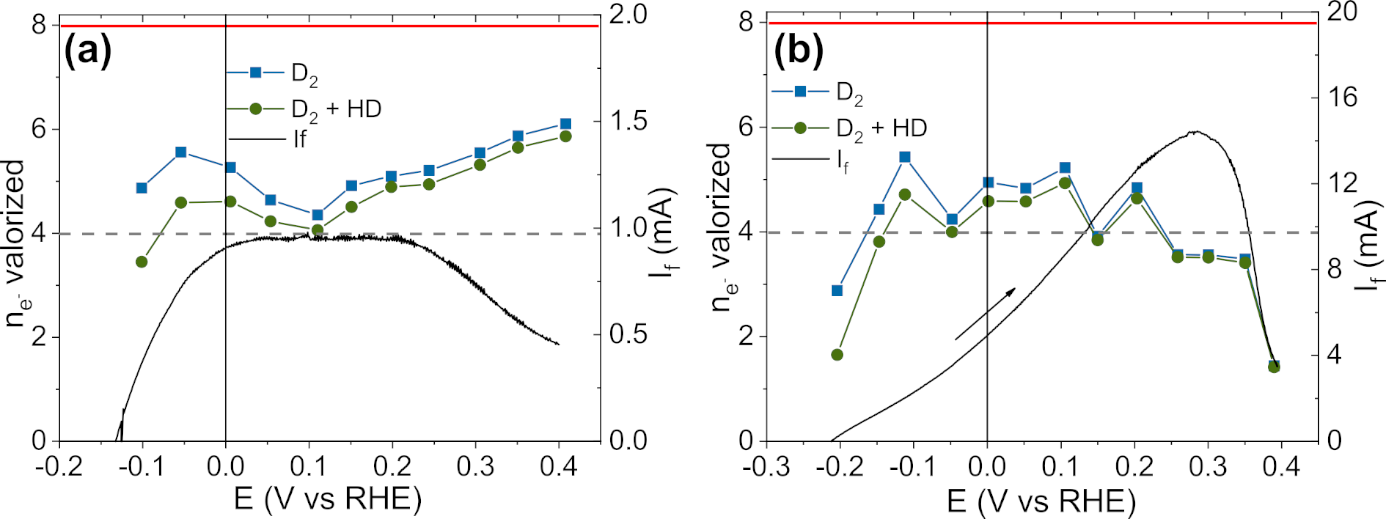
So, the NaBH4 oxidation reaction (BOR) was studied on nickel surfaces, in collaboration with ICPEES, Université of Strasbourg and University of Pennsylvania (USA). Combining in situ Fourier-transform IR-spectroscopy (FTIR), differential electrochemical mass-spectrometry (DEMS) and density functional theory (DFT) calculations, the BOR mechanisms on smooth Ni electrodes was fully unveiled 22; notably, the BOR on Ni produces 4 instead of 8 e −in theory and is accompanied by H2 formation (Figure 1).
From this model study, more practical 3D-textured Ni electrodes have been studied and optimized, in collaboration with LEMTA, Université de Lorraine 23-25. Notably, open structures like a Ni felt (very porous to let H2 bubbles escapes preserving access to the electrolyte and fuel) lead to very high activity if their state of surface is metallic (after etching) and their developed surface area large (after electrodeposition) (Figure 2).
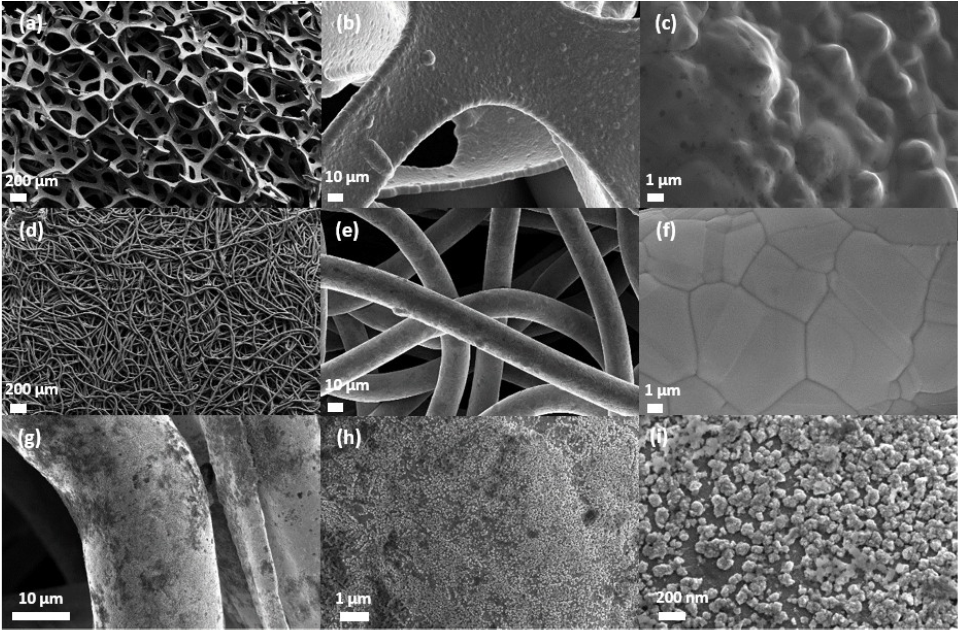
With such NiED/NFT electrodes, Pt-based and nickel-based electrodes supported on classical carbon-based gas diffusion layers (of much more closed structure) are largely outperformed (Figure 3).
LEPMI-EIP has also opened such studies on Ni-based electrodes to other fuels, of the borane family 26 and hydrazine 27,28.
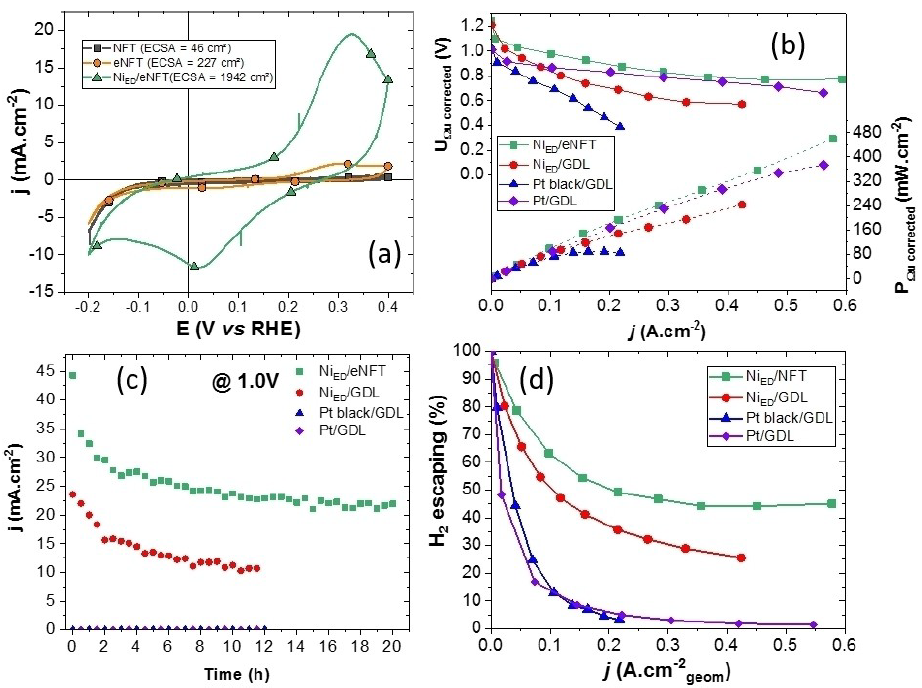
Lastly, combining such NiED/NFT electrodes with bipolar membranes (Georges Washington University, Saint Louis, USA) enables high performance in direct borohydride fuel cells using hydrogen peroxide as the oxidant 29.
Hollow and carbon-capped PtNi nanoparticles for the oxygen reduction reaction in PEMFCs operating at 95°C
For heavy-duty applications (trucks, buses, trains, planes), that are now targeted for PEMFCs, it is important to raise the stack operating temperature, so to enable easier stack cooling and humidification. However, higher operating temperatures accelerate the degradation of the PEMFC core materials, notably the electrocatalyst. In that frame, the LEPMI-EIP team designs, elaborates and validates PEMFC cathode catalysts that are able to durably operate at higher temperature, 95°C being a clear objective. In these conditions, dissolution and agglomeration of the nanoparticles are frequently-encountered degradation mechanisms of usual Pt(M)/C catalysts, making the discovery of more robust catalyst a mandatory approach, without of coursed compromising their activity for the oxygen reduction reaction (ORR).In a first approach, Pt sponge/hollow particles, initially “discovered” from the degradation of Pt3Co/C alloys in PEMFC on-site operation, were elaborated and tested for their ORR activity and durability in PEMFC environment. In principle these catalysts are extremely active -the best porous hollow PtNi/C nanocatalysts achieve 6-fold and 9-fold enhancement in mass and specific activity for the ORR, respectively over standard solid Pt/C nanocrystallites of the same size 30. The better catalytic activity for the ORR of porous hollow PtNi/C nanocatalysts is ascribed to (i) their opened porosity, (ii) their preferential crystallographic orientation (“ensemble effect”), and (iii) the weakened oxygen binding energy induced by the contracted Pt lattice parameter (“strain effect”). However, these promises (measured in rotating disk electrode – model configuration) are not always transferred into PEMFC conditions, notably because the classical catalytic layer design is not appropriate for the integration of such catalysts (that usually present low surface area and very active sites). Within the BRIDGE project, LEPMI-EIP and its partners strived to optimize the catalytic layer design 31, but also found that these advanced defective catalysts were prone to non-negligible dissolution of their Pt and Ni components 32. Another issue related to these advanced catalysts, is that they operate well in PEMFC only at low current density (Figure 1). At higher current densities, their low developed surface area (low amount of active sites) combined with the very high activity of these active sites makes it complex to feed each active site appropriately by protons and O2 molecules, emphasizing the mass-transport loss 31. To cope with this issue, within the PEMFC95 project, the sponge PtNi/C catalysts are now further studied, to increase their developed surface area and facilitate their implementation in efficient PEMFC catalytic layers.
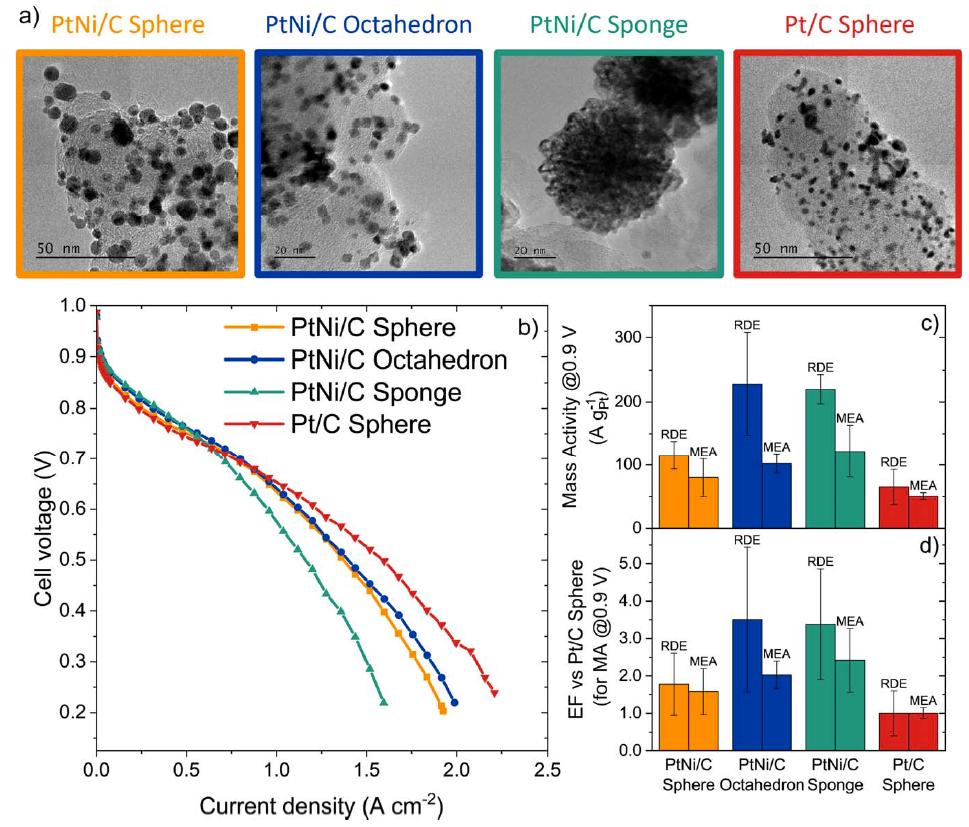
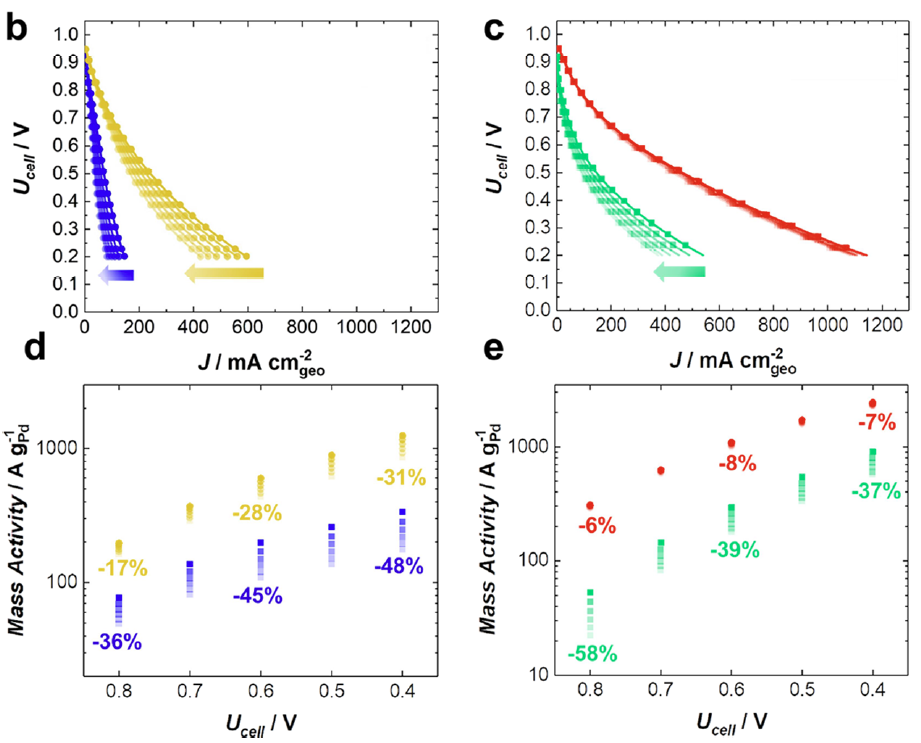
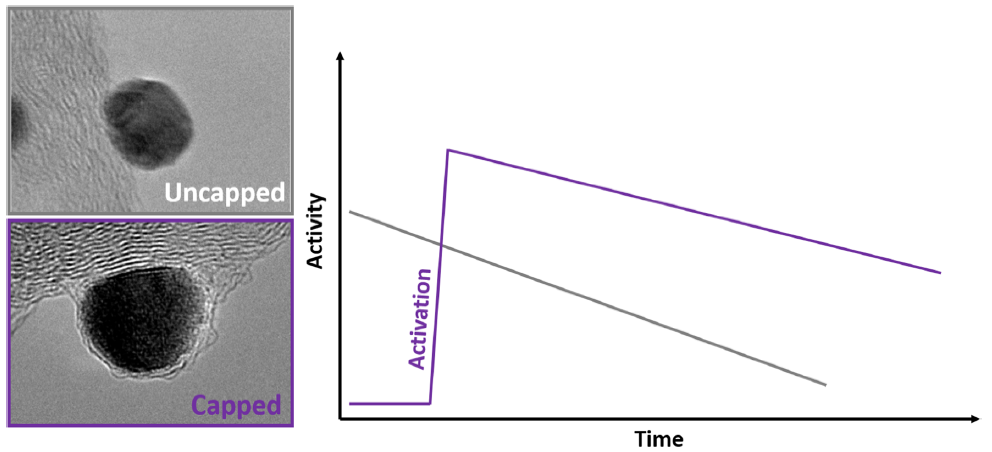
In a second approach, the PtNi nanoparticles (that are supported on the conductive carbon substrate) are protected by a carbon cap, that should slow down the degradations, ideally without compromising their instrinsic ORR activity. The carbon cap should on the one hand be protective versus the degradation phenomena, but also porous to enable access to the H+ and O2 reactants. This research is conducted within the PEMFC95 project in close collaboration with CEA-LITEN. The carbon cap indeed inhibits the metal dissolution (to some extent) and the nanoparticles migration (Figure 2), rendering the PtNi@C/C catalysts more robust than classical Pt/C catalysts, without compromising the superior activity brought by the Ni alloying to Pt. However, more work is needed to integrate these catalysts in efficient PEMFC catalytic layers and improve their intrinsic ORR properties, which requires a proper activation of the carbon cap 33.
Acknowledgements
This work is performed within the framework of the Centre of Excellence of Multifunctional Architectured Materials "CEMAM" n° AN-10-LABX-44-01 funded by the "Investments for the Future" program. The authors acknowledge financial support from the French National Research Agency through the BRIDGE project (grant number ANR-19-ENER-0008-01) and the PEMFC95 project, funded by France 2030 via PEPR-H2 (ANR-22-PEHY-0005).References
1 Chatenet, M., Micoud, F., Roche, I., Chainet, E. & Vondrak, J. Kinetics of sodium borohydride direct oxidation and oxygen reduction in sodium hydroxide electrolyte - Part II. O-2 reduction. Electrochim. Acta 51, 5452-5458 (2006).
2 Roche, I., Chainet, E., Chatenet, M. & Vondrak, J. Carbon-supported manganese oxide nanoparticles as electrocatalysts for the Oxygen Reduction Reaction (ORR) in alkaline medium: Physical characterizations and ORR mechanism. J. Phys. Chem. C 111, 1434-1443 (2007).
3 Roche, I., Chainet, E., Vondrak, J. & Chatenet, M. Durability of carbon-supported manganese oxide nanoparticles for the oxygen reduction reaction (ORR) in alkaline medium. J. Appl. Electrochem. 38, 1195-1201 (2008).
4 Garcia, A. C., Herrera, A. D., Ticianelli, E. A., Chatenet, M. & Poinsignon, C. Evaluation of Several Carbon-Supported Nanostructured Ni-Doped Manganese Oxide Materials for the Electrochemical Reduction of Oxygen. J. Electrochem. Soc. 158, B290-B296 (2011).
5 Garcia, A. C., Lima, F. H. B., Ticianelli, E. A. & Chatenet, M. Carbon-supported nickel-doped manganese oxides as electrocatalysts for the oxygen reduction reaction in the presence of sodium borohydride. J. Power Sources 222, 305-312 (2013). https://doi.org:10.1016/j.jpowsour.2012.08.049
6 Moureaux, F., Stevens, P. & Chatenet, M. Effect of Lithium and Potassium Cations on the Electrocatalytic Properties of Carbon and Manganese Oxide Electrocatalysts Towards the Oxygen Reduction Reaction in Concentrated Alkaline Electrolyte. Electrocatal. 4, 123-133 (2013). https://doi.org:10.1007/s12678-013-0127-4
7 Garcia, A., Linares, J., Chatenet, M. & Ticianelli, E. NiMnOx/C: A Non-noble Ethanol-Tolerant Catalyst for Oxygen Reduction in Alkaline Exchange Membrane DEFC. Electrocatal. 5, 41-49 (2014). https://doi.org:10.1007/s12678-013-0162-1
8 Sgarbi, R. et al. Oxygen reduction reaction mechanism and kinetics on M-NxCy and M@N-C active sites present in model M-N-C catalysts under alkaline and acidic conditions. J. Solid State Electrochem. 25, 45-56 (2021). https://doi.org:10.1007/s10008-019-04436-w
9 Zadick, A., Dubau, L., Zalineeva, A., Coutanceau, C. & Chatenet, M. When cubic nanoparticles get spherical: An Identical Location Transmission Electron Microscopy case study with Pd in alkaline media. Electrochem. Commun. 48, 1-4 (2014). https://doi.org:http://dx.doi.org/10.1016/j.elecom.2014.07.020
10 Zadick, A., Dubau, L., Sergent, N., Berthomé, G. & Chatenet, M. Huge Instability of Pt/C Catalysts in Alkaline Medium. ACS Catal. 5, 4819-4824 (2015). https://doi.org:10.1021/acscatal.5b01037
11 Zadick, A., Dubau, L., Demirci, U. B. & Chatenet, M. Effects of Pd Nanoparticle Size and Solution Reducer Strength on Pd/C Electrocatalyst Stability in Alkaline Electrolyte. J. Electrochem. Soc. 163, F781-F787 (2016). https://doi.org:10.1149/2.0141608jes
12 Lafforgue, C., Zadick, A., Dubau, L., Maillard, F. & Chatenet, M. Selected review of the degradation of Pt and Pd-based carbonsupported electrocatalysts for alkaline fuel cells: towards mechanisms of degradation. Fuel Cells 18, 229-238 (2018). https://doi.org:10.1002/fuce.201700094
13 Sgarbi, R., Doan, H., Martin, V. & Chatenet, M. Tailoring the Durability of Carbon-Coated Pd Catalysts Towards Hydrogen Oxidation Reaction (HOR) in Alkaline Media. Electrocatal. 14, 267-278 (2023). https://doi.org:10.1007/s12678-022-00794-8
14 Sgarbi, R. et al. Improved Activity and Durability of Carbon-Capped Pd and PdNi CatalystsFrom Model Active Layers to Anion-Exchange Membrane Fuel Cell Electrodes. ACS Catal. 15, 9379−9392 (2025). https://doi.org:https://doi.org/10.1021/acscatal.5c00704
15 Braesch, G., Bonnefont, A., Martin, V., Savinova, E. R. & Chatenet, M. Borohydride oxidation reaction mechanisms and poisoning effects on Au, Pt and Pd bulk electrodes: From model (low) to direct borohydride fuel cell operating (high) concentrations. Electrochim. Acta 273, 483-494 (2018). https://doi.org:https://doi.org/10.1016/j.electacta.2018.04.068
16 Olu, P.-Y. et al. Influence of the concentration of borohydride towards hydrogen production and escape for borohydride oxidation reaction on Pt and Au electrodes – experimental and modelling insights. J. Power Sources 375, 300-309 (2018). https://doi.org:https://doi.org/10.1016/j.jpowsour.2017.07.061
17 Olu, P., Zadick, A., Job, N. & Chatenet, M. in Electrocatalysts for Low Temperature Fuel Cells: Fundamentals and Recent Trends 317-346 (2017).
18 Zadick, A. et al. Ubiquitous Borane Fuel Electrooxidation on Pd/C and Pt/C Electrocatalysts: Toward Promising Direct Hydrazine–Borane Fuel Cells. ACS Catal. 8, 3150-3163 (2018). https://doi.org:10.1021/acscatal.7b04321
19 Pylypko, S. et al. The highly stable aqueous solution of sodium dodecahydro-closo-dodecaborate Na2B12H12 as a potential liquid anodic fuel. Appl. Catal. B: Environmental 222, 1-8 (2018). https://doi.org:10.1016/j.apcatb.2017.09.068
20 Lafforgue, C., Chatenet, M., Dubau, L. & Dekel, D. R. Accelerated Stress Test of Pt/C Nanoparticles in an Interface with an Anion-Exchange Membrane—An Identical-Location Transmission Electron Microscopy Study. ACS Catal. 8, 1278-1286 (2018). https://doi.org:10.1021/acscatal.7b04055
21 Lafforgue, C., Maillard, F., Martin, V., Dubau, L. & Chatenet, M. Degradation of Carbon-supported Platinum Group Metal Electrocatalysts in Alkaline Media Studied by in situ Fourier-Transform Infrared Spectroscopy and Identical-Location Transmission Electron Microscopy. ACS Catal. 9, 5613−5622 (2019). https://doi.org:10.1021/acscatal.9b00439
22 Oshchepkov, A. G. et al. Insights into the borohydride electrooxidation reaction on metallic nickel from operando FTIRS, on-line DEMS and DFT. Electrochim. Acta 389, 138721 (2021). https://doi.org:https://doi.org/10.1016/j.electacta.2021.138721
23 Oshchepkov, A. G. et al. Nickel Metal Nanoparticles as Anode Electrocatalysts for Highly Efficient Direct Borohydride Fuel Cells. ACS Catal. 9, 8520-8528 (2019). https://doi.org:10.1021/acscatal.9b01616
24 Braesch, G. et al. Nickel 3D Structures Enhanced by Electrodeposition of Nickel Nanoparticles as High Performance Anodes for Direct Borohydride Fuel Cells. ChemElectroChem 7, 1789-1799 (2020). https://doi.org:10.1002/celc.202000254
25 Oshchepkov, A. G., Braesch, G., Bonnefont, A., Savinova, E. R. & Chatenet, M. Recent Advances in the Understanding of Nickel-Based Catalysts for the Oxidation of Hydrogen-Containing Fuels in Alkaline Media. ACS Catal. 10, 7043-7068 (2020). https://doi.org:10.1021/acscatal.0c00101
26 Zadick, A. et al. Nickel-based electrocatalysts for ammonia borane oxidation: enabling materials for carbon-free-fuel direct liquid alkaline fuel cell technology. Nano Energy 37, 248-259 (2017). https://doi.org:https://doi.org/10.1016/j.nanoen.2017.05.035
27 Vorms, E. A., Oshchepkov, A. G., Bonnefont, A., Savinova, E. R. & Chatenet, M. Carbon-free fuels for direct liquid-feed fuel cells: Anodic electrocatalysts and influence of the experimental conditions on the reaction kinetics and mechanisms. Appl. Catal. B: Environmental 345, 123676 (2024). https://doi.org:https://doi.org/10.1016/j.apcatb.2023.123676
28 Vorms, E. A. et al. Mechanism of the hydrazine hydrate electrooxidation reaction on metallic Ni electrodes in alkaline media as revealed by electrochemical methods, online DEMS and ex situ XPS. Electrochim. Acta 507, 145056 (2024). https://doi.org:https://doi.org/10.1016/j.electacta.2024.145056
29 Braesch, G. et al. A high performance direct borohydride fuel cell using bipolar interfaces and noble metal-free Ni-based anodes. J. Mater. Chem. A 8, 20543 - 20552 (2020). https://doi.org:10.1039/d0ta06405j
30 Dubau, L. et al. Tuning the Performance and the Stability of Porous Hollow PtNi/C Nanostructures for the Oxygen Reduction Reaction. ACS Catal. 5, 5333-5341 (2015). https://doi.org:10.1021/acscatal.5b01248
31 Roiron, C. et al. Tracking the Early-life of PtNi/C Shape-Controlled Catalysts upon their Integration in PEMFC. J. Electrochem. Soc. 171, 114504 (2024). https://doi.org:10.1149/1945-7111/ad8d7f
32 Roiron, C., Martin, V., Kumar, K., Dubau, L. & Maillard, F. Assessing Pt and Ni dissolution mechanism and kinetics of shape-controlled oxygen reduction nanocatalysts. Electrochim. Acta 477 (2024). https://doi.org:10.1016/j.electacta.2024.143760
33 Labarde, Q., Godoy, A. O., Dubau, L., Micoud, F. & Chatenet, M. Carbon-Capped PtNi Catalysts for the Oxygen Reduction Reaction in Acidic Environment: A Durability Study. Electrocatal. 16, 117-131 (2025). https://doi.org:10.1007/s12678-024-00904-8


