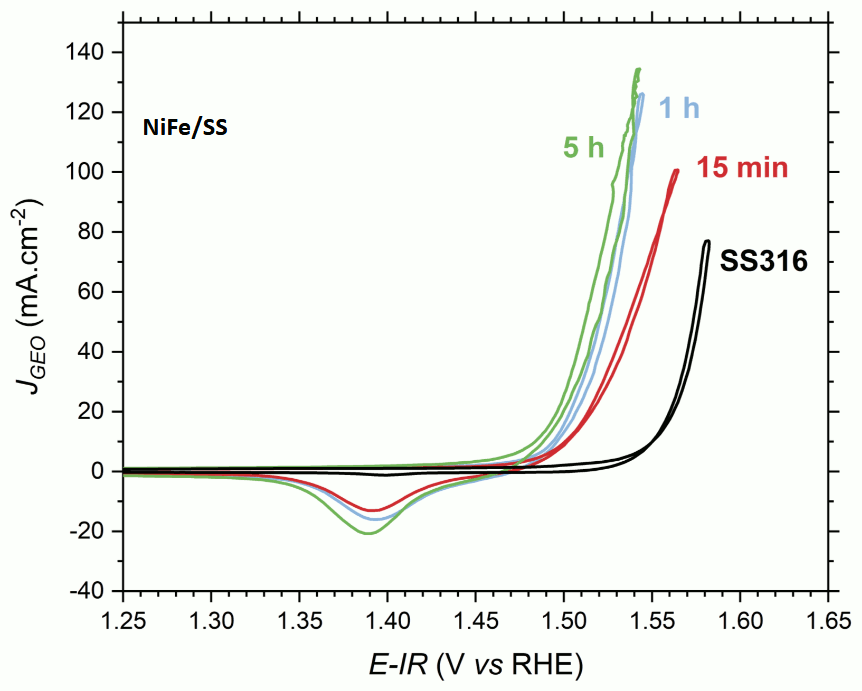
As part of the water electrolysis process to produce carbon-free hydrogen, we are working on developing electrocatalysts that are both effective and stable over time, for both oxygen evolution (OER) and hydrogen evolution (HER) reactions. Various physical and/or chemical characterisation methods are used to understand the basic mechanisms of gas evolution reactions and electrode ageing in order to improve the systems. Work is being carried out on the three main technologies under consideration. For alkaline water electrolysis (AWE), research is focusing on the nanostructuring of electrodes to promote the formation and detachment of gas bubbles. For proton exchange membrane water electrolysis (PEMWE), the aim is to reduce the use of precious metals (platinum and iridium [2]) and to recycle them. Finally, for alkaline membrane electrolysis (AEMWE: Anion Exchange Membrane Water Electrolyser), work is focusing on the use of NiFe alloy-based catalysts [1].
 |
 |
 |
The team is involved in the PEPR-H2 DAEMONHYC project, in collaboration with ICPEES in Strasbourg, LEMTA in Nancy, LPPI in Cergy-Pontoise, and companies GenHy and Air Liquide. We also have a collaboration with Adele Hydrogen.
Main publications :
- [1] L. Magnier, G. Cossard, V. Martin, C. Pascal, V. Roche, E. Sibert, I. Shchedrina, R. Bousquet, V. Parry, M. Chatenet, Fe–Ni-based alloys as highly active and low-cost oxygen evolution reaction catalyst in alkaline media, Nat. Mater. (2024) 1–10. https://doi.org/10.1038/s41563-023-01744-5.
- [2] M. Scohy, S. Abbou, V. Martin, B. Gilles, E. Sibert, L. Dubau, F. Maillard, Probing Surface Oxide Formation and Dissolution on/of Ir Single Crystals via X-ray Photoelectron Spectroscopy and Inductively Coupled Plasma Mass Spectrometry, ACS Catal. 9 (2019) 9859–9869. https://doi.org/10.1021/acscatal.9b02988.



