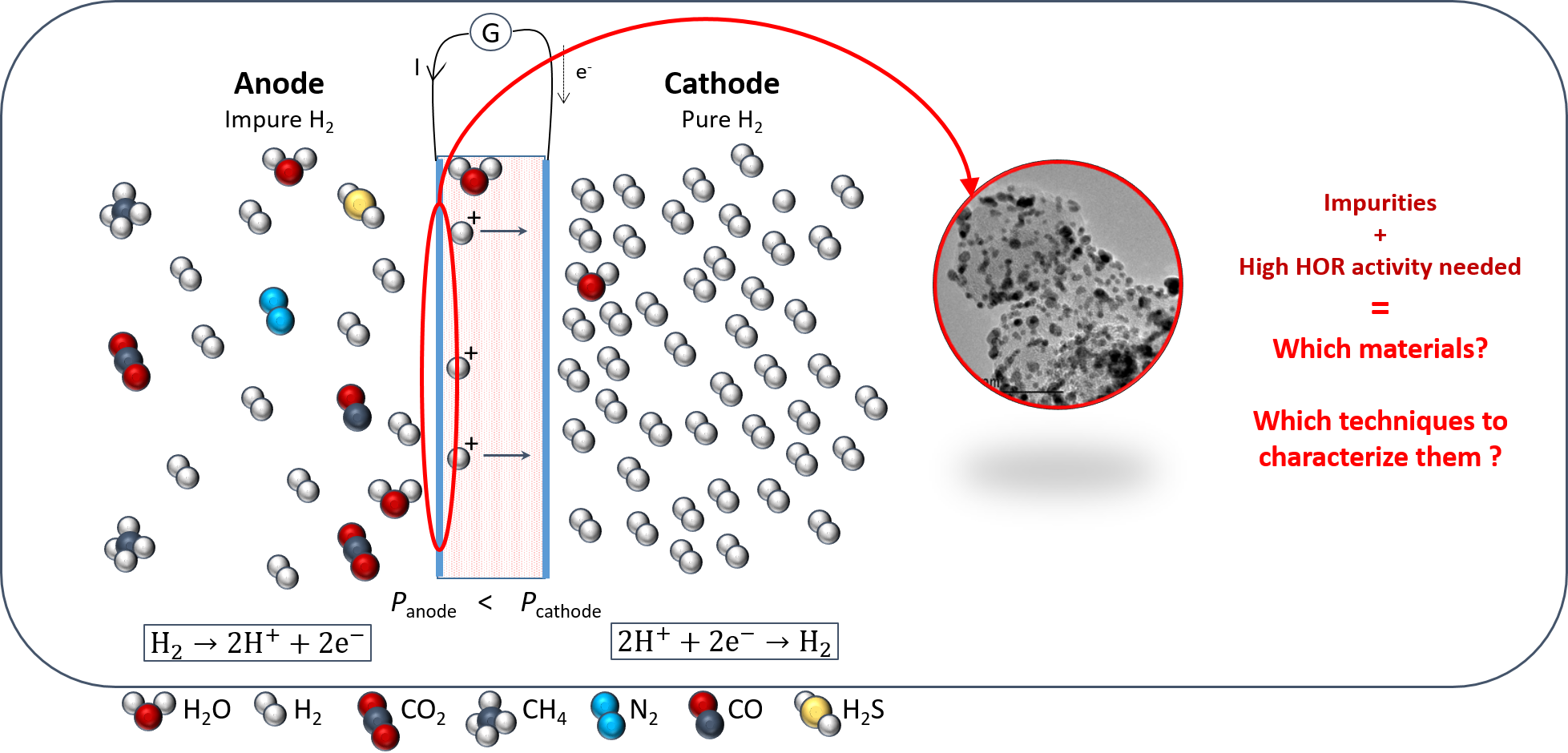
The electrochemical hydrogen compressor (EHC) is a promising system combining both the purification and compression steps required for the use of hydrogen in devices such as proton exchange membrane fuel cells. Its usage could enable to reduce the price of hydrogen, thus facilitating the popularization of hydrogen-powered devices. However, to be competitive, EHC must sustain large current densities (in the range of 2 A cm-2), an ambitious goal considering the presence of pollutants gases at the anode, where the hydrogen oxidation reaction (HOR) is performed. Therefore, it is essential for the anode electrocatalysts to present a high tolerance towards poisoning impurities such as CO or CO2, as well as very good activity towards the HOR.
The present work investigates two families of electrocatalysts with promising properties in those aspects. On the one hand, the Pt+Ru family, composed of unalloyed Pt and Ru nanoparticles deposited on carbon support, presented CO-oxidation current at potential values as low as for pure Ru, while keeping a good activity for the HOR when measured in rotating disk electrode (contrary to Ru, that displays poor HOR activity). On the other hand, the WO3-supported Pt and Pt+Ru electrocatalysts displayed good CO-oxidation onset, despite a lower HOR activity and a very poor stability. The most promising electrocatalysts were compared in a gas diffusion electrode (GDE) test cell, in which mass transport limitations are lowered. This enabled the proper comparison of all the electrocatalysts at high current densities, an endeavour to their use in EHC.
Direction/co-direction : M. Marian CHATENET, M. Christophe GEANTET
Composition du jury :
Mme Sophie DIDIERJEAN Université de Lorraine Rapporteure
M. Pierre MILLET Université Paris Saclay Rapporteur
M. Gaël MARANZANA Université de Lorraine Examinateur
Mme Laetitia DUBAU Grenoble INP Examinatrice
Infos date
Defense 16 July 2021 at 9H30
Amphithéâtre Jean Besson (Bâtiment Phelma Campus)