Degradation mechanisms of non-PGM catalysts for oxygen reduction reaction in acidic media
A possible path to solve the economic and geological constraints associated with the use of Pt consists in developing non-precious metals catalysts (NPMCs). Transition metal oxides, nitrides and/or carbides,transition metal chalcogenides, nanostructured carbon materials, nitrogen-, sulphur-, boron-, phosphorous-doped carbon materials, and transition metal-nitrogen-carbon catalysts have proven highly active for the ORR in acid and alkaline electrolyte. Among NPMCs, the most mature sub-class of materials is prepared via the pyrolysis of metal atoms (Fe or Co), nitrogen (N) and carbon (C) precursors. Their initial ORR activity strongly depends on their physical and chemical structure, namely the nature and coordination of the metal centres, metal content, redox potential, basicity of N-containing groups and pore size distribution in the carbon phase.
The ORR performance of Metal-N-C catalysts often decreases during ageing in acidic medium both in rotating-disk electrode (RDE) and membrane|electrode assembly set-ups, but the underlying degradation mechanisms remain under-investigated. To gain some insights into the underlying degradation mechanism, we use Metal-N-C catalysts prepared in the group of Frédéric Jaouen in ICGM, differing from each other by the nature of the metal (Fe or Co), the metal content and the heating mode applied during pyrolysis that we characterize before after ageing in conditions simulating PEMFC cathode operating conditions (80°C, load-cycling or start-up/shutdown protocol, 10 k cycles). By combining the information derived from X-ray absorption, Raman and energy-dispersive X-ray spectroscopies and electrochemistry, we conclude that demetallation of the catalytic centres and corrosion of the carbon matrix are one of the reasons of ORR activity decay during load-cycling and start-up/shutdown cycling under Ar-atmosphere, respectively. To know more on this.
A possible path to solve the economic and geological constraints associated with the use of Pt consists in developing non-precious metals catalysts (NPMCs). Transition metal oxides, nitrides and/or carbides,transition metal chalcogenides, nanostructured carbon materials, nitrogen-, sulphur-, boron-, phosphorous-doped carbon materials, and transition metal-nitrogen-carbon catalysts have proven highly active for the ORR in acid and alkaline electrolyte. Among NPMCs, the most mature sub-class of materials is prepared via the pyrolysis of metal atoms (Fe or Co), nitrogen (N) and carbon (C) precursors. Their initial ORR activity strongly depends on their physical and chemical structure, namely the nature and coordination of the metal centres, metal content, redox potential, basicity of N-containing groups and pore size distribution in the carbon phase.
The ORR performance of Metal-N-C catalysts often decreases during ageing in acidic medium both in rotating-disk electrode (RDE) and membrane|electrode assembly set-ups, but the underlying degradation mechanisms remain under-investigated. To gain some insights into the underlying degradation mechanism, we use Metal-N-C catalysts prepared in the group of Frédéric Jaouen in ICGM, differing from each other by the nature of the metal (Fe or Co), the metal content and the heating mode applied during pyrolysis that we characterize before after ageing in conditions simulating PEMFC cathode operating conditions (80°C, load-cycling or start-up/shutdown protocol, 10 k cycles). By combining the information derived from X-ray absorption, Raman and energy-dispersive X-ray spectroscopies and electrochemistry, we conclude that demetallation of the catalytic centres and corrosion of the carbon matrix are one of the reasons of ORR activity decay during load-cycling and start-up/shutdown cycling under Ar-atmosphere, respectively. To know more on this.

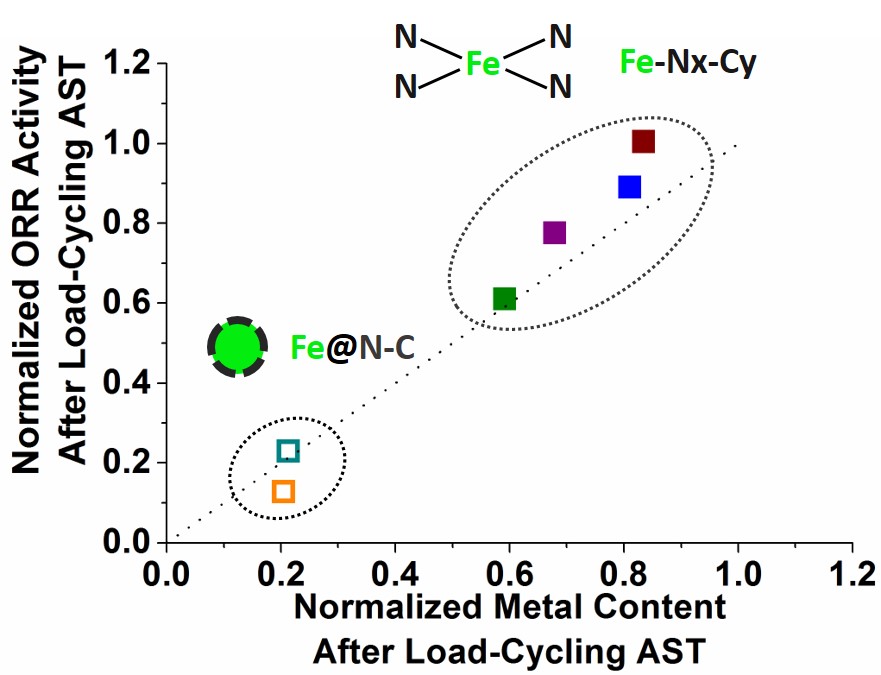
Figure 1. Demetallation of the catalytic centres is the main cause of the decrease in ORR activity of Metal-N-C electrocatalysts during load-cycling under neutral atmosphere.
Acknowledgements
This work is performed within the framework of the Centre of Excellence of Multifunctional Architectured Materials “CEMAM” n° AN-10-LABX-44-01. The authors acknowledge financial support from the French National Research Agency through the CAT2CAT and MOISE projects.
References
1. K. Kumar, P. Gairola, M. Lions, N. Ranjbar-Sahraie, M. Mermoux, L. Dubau, A. Zitolo, F. Jaouen, F. Maillard, “Physical and Chemical Considerations for Improving Catalytic Activity and Stability of Non-Precious Metal Oxygen Reduction Reaction Catalysts”, ACS Catal. 8 (2018) 11264-11276. DOI: 10.1021/acscatal.8b02934
2. R. Sgarbi, K. Kumar, F. Jaouen, A. Zitolo, E. Ticianelli, F. Maillard, “Oxygen Reduction Reaction Mechanism and Kinetics on M-NxCy and M@N-C Active Sites Present in Model M-N-C Catalysts Under Alkaline and Acidic Conditions”, J. Solid State Electrochem., invited article in honour of the 70th birthday of Prof. Dr. José Zagal.
3. K. Kumar, L. Dubau, M. Mermoux, J. Li. A. Zitolo, J. Nelayah, F. Jaouen, F. Maillard, “On the Influence of Oxygen on the Degradation of Fe-N-C Catalysts”, (2019) accepted in Angew. Chemie. Int. Ed. DOI: 10.1002/anie.201912451
This work is performed within the framework of the Centre of Excellence of Multifunctional Architectured Materials “CEMAM” n° AN-10-LABX-44-01. The authors acknowledge financial support from the French National Research Agency through the CAT2CAT and MOISE projects.
References
1. K. Kumar, P. Gairola, M. Lions, N. Ranjbar-Sahraie, M. Mermoux, L. Dubau, A. Zitolo, F. Jaouen, F. Maillard, “Physical and Chemical Considerations for Improving Catalytic Activity and Stability of Non-Precious Metal Oxygen Reduction Reaction Catalysts”, ACS Catal. 8 (2018) 11264-11276. DOI: 10.1021/acscatal.8b02934
2. R. Sgarbi, K. Kumar, F. Jaouen, A. Zitolo, E. Ticianelli, F. Maillard, “Oxygen Reduction Reaction Mechanism and Kinetics on M-NxCy and M@N-C Active Sites Present in Model M-N-C Catalysts Under Alkaline and Acidic Conditions”, J. Solid State Electrochem., invited article in honour of the 70th birthday of Prof. Dr. José Zagal.
3. K. Kumar, L. Dubau, M. Mermoux, J. Li. A. Zitolo, J. Nelayah, F. Jaouen, F. Maillard, “On the Influence of Oxygen on the Degradation of Fe-N-C Catalysts”, (2019) accepted in Angew. Chemie. Int. Ed. DOI: 10.1002/anie.201912451



