Activity overview
The electrochemical reactors have been used for more than a century in much industry. The metal production such as aluminum production or gas production such as chlorine production are the main industries that use electrochemical reactors. Nowadays, in the context of the spread of renewable energies the electrochemical devices have been successful applied to energy storage (e.g. power to gas). Moreover, the electrochemical technics are powerful tools of characterization for investigation of physical phenomena (e.g. electroanalytical technics, conductimetry, impedance meter, sensors etc.).
The core of the electrochemical cells is the electrochemistry. Furthermore, the electrokinetitcs at the surface reaction involves charge and mass transfer and it depends largely on the operating conditions. The performance of the electrochemical reaction requires the control of all the phenomena involved. Many physical processes have a strong impact on the behavior and performance of electrochemical devices. Whether it is the nature of the electrolyte (solid, liquid, flowing, multiphase) or electrode structure (porous, architectured materials) or the combination with other physical phenomena (thermal, magnetic).
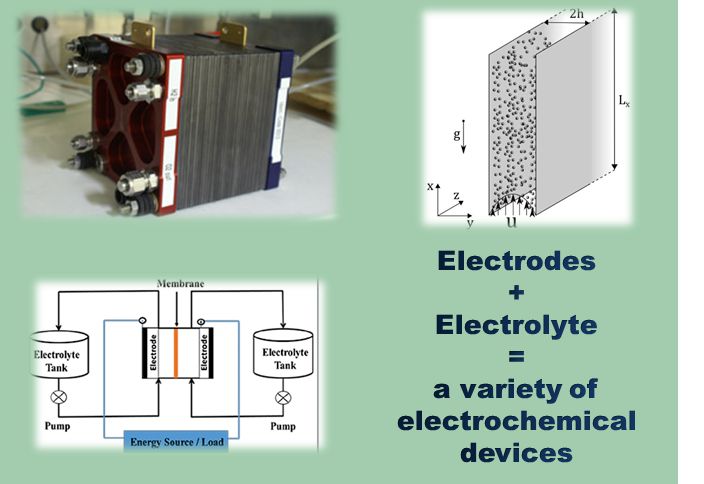
Electrochemical reactor
Relevant approach to design and optimize the electrochemical devices is multiphysic approach. Our group adopts this approach to study electrochemical devices from a fundamental point of view and in different industrial applications.
Among issues addressed in this framework are:
- Electrochemistry and Magnetic Field
- Multiphase Flow in electrochemical cell
- Modeling tools for characterisation and design
Studies can focus on different electrochemical cells and are carried out in collaboration with other groups of our institute for electrochemistry material (LMOPS, MIEL), electrocatalysis (EIP), durability and recyclability (EIP) :
- Proton Exchange Membrane device (PEMFC, PEMWE, electrochemical compressor), partnership : LE2P, LEMTA;
Associated program : ANR LOCALI, CDP ECOsesa - Redox-flow battery, partnership : MIEL, LGC;
Associated program : ANR VSL - Alkaline eltrolysis (AWE), patnership : LPCNO;
Associated program : ANR Hy-walHy - Biological and electrochemical system for hydrogen production.
Associated program : Hystep - Hydrogen technologies, purification, dissociated electrolysis industrial Partnership : ERGOSUP;
Associated program : Hypper
Electrochemistry and Magnetic Field
Durability of PEMFC system is “the worry” of EIP group, and then to develop new strategy to detect the degradation is a continuous endeavor.
Many phenomena such as MEA degradation, membrane drying and electrode flooding affect the current density distribution. For example, the measurement of magnetic fields allows for the building of a three dimensional picture of the internal current density possible. The magnetotomography can supply a lot of information about the operating conditions within a fuel cell. It is possible to estimate an averaged 2D current density identification by solving an inverse problem, based on the relationship between the currents and a magnetic field. Therefore to solve an inverse problem, we need a “good” direct model”. The model of stack coupled to magnetic sensor highlights the nonhomogeneity of current density through the cell and between the cells of the stack. This methodology is performed in the ANR program LOCALI (Projet-ANR-17-CE05-0003)
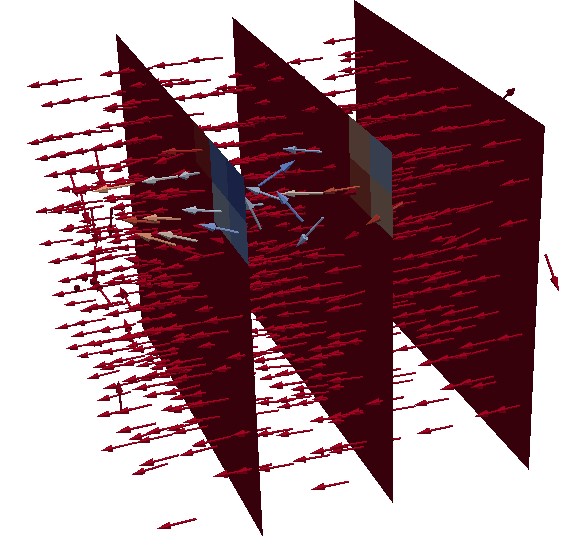
Magnetic field on PEMFC
Enhance electrocatalysis of AWE without using PGM catalysts is a grand challenge that EIP group has always addressed.
We have proved that the magnetic heating effect can be applied to improve the activity of a FeC@Ni catalyst for the reactions of water electrolysis in 1 mol L-1 KOH: The OER overpotential during galvanostatic measurements could be decreased by up to 200 mV in a 40 mT electromagnetic field, in a range of current density from 10 to 25 mA cm-2. Furthermore, only at “high” current density up to 25 mA cm-2, the hydrogen evolution reaction (HER) exhibited a decrease in overpotential up to 75 mV in a 48 mT electromagnetic field. Therefore, with magnetic heating, to attain the productivity of PEMWE of 1 A cm-2 with less than 500 mV of electrode overvoltages. The Hy-walHy ANR program (ANR-17-CE05-0017) proposes to develop magnetically-enhanced alkaline water electrolyzers (AWE), on which LEPMI and LPCNO have recently established a proof-of-concept.
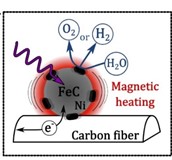
Alternative Magnetic Field on Alkaline Water electrolyzer
Electrochemistry and Multiphase flow
In numerous electrochemistry device, we are confronted to multiphase flow. Solids particles, droplets or bubble, uncharged species could present and should have a strong impact on mass and charge transfer at interface. Furthermore, electrochemical reactions can be enhanced by forced electrolyte flow, which increases mass transfer at the electrode surface. Alternatively, a flow can be induced by the electrochemical bubble production. Hence, the efficiency of the electrochemical processes strongly depends on mass transfer.
EIP develop approaches that could be valid to numerous cases of multiphasic flow.
Liquid/gas multiphase induced flow
In alkaline water electrolysis process the additional resistance result from partial coverage of the electrodes by the bubbles was critical in energy efficiency. A better understanding of bubble behavior would provide a scientific guidance to minimize this resistance and contribute to the development of hydrogen production. The developed approach in EIP group shows, that it is possible to predict the evolution of the bubble plumes along the electrodes in free and forced convection. Using this results it is possible to optimize the operation of electrolyze by means of an analysis of classical dimensionless numbers such as Reynolds (forced) or Rayleigh (free) “like” number and equivalent Prandtl number.
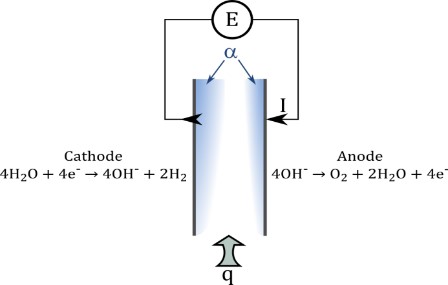
Liquid/gas multiphase induced flow
Liquid/solid multiphase forced flow
In the V.S.L ANR program (VSL-Projet-ANR-17-CE05-0023) studies aims to enhance energy and power density of all vanadium redox-flow battery. This Program investigate an innovative design Vanadium-Liquid-Solid cell. A lab scale cell (single flow cell) is used to study electrolyte behavior as well as membrane behavior for this specific multiphase flow electrolyte. The presence of a solid phase influences behavior and performance such as bubbles.
Single electrochemical cell for flow electrolyte.
Modelling tools for characterization and design
Electrochemical Impedance Spectroscopy
EIS is a recognized diagnosis tools. Frequently, performing accurate and reliable measurement of the polarization resistances and electrolyte behavior that is an important step for a lot of applications in the applied electrochemistry. The placement of working electrode, counter electrode and reference electrode in solid ionic conductor or multiphasic electrolyte as less flexible as usual ionic liquid. It is also noticed that a non-symmetrical arrangement of RE and CE leads to significant errors on the recorded WE impedance. The simulations can be used to predict the deviation of electrical measurement from the real electrode impedance contribution and the computing can investigate that an optimum placement of RE exists for a fixed cell configuration.
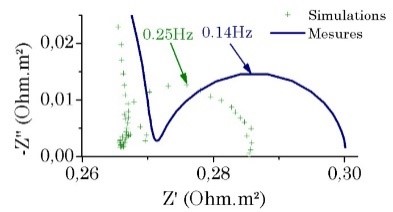
EIS spectra.
Modelling of transport and transfer
One-dimensional mathematical models are developed by means of charge and mass transport balances in Membrane Electrode Assembly (AME). This approach allows to obtain analytical solutions of the water content at the membrane, the over potential and the current density distribution at the membrane and the catalyst layers and offered a useful tool for the comprehension of the electrochemical device such as electrolyser, electrochemical compressor. Electrochemical compressor modelling is included in Eco-SESA CDP. One of the main idea is to develop a reliable and energy-efficient hydrogen purifier/compressor operating at low temperature, compatible for an eco-district. Modelling works and study of electrocatalyst suitable for purification are carried out in parallel by EIP.
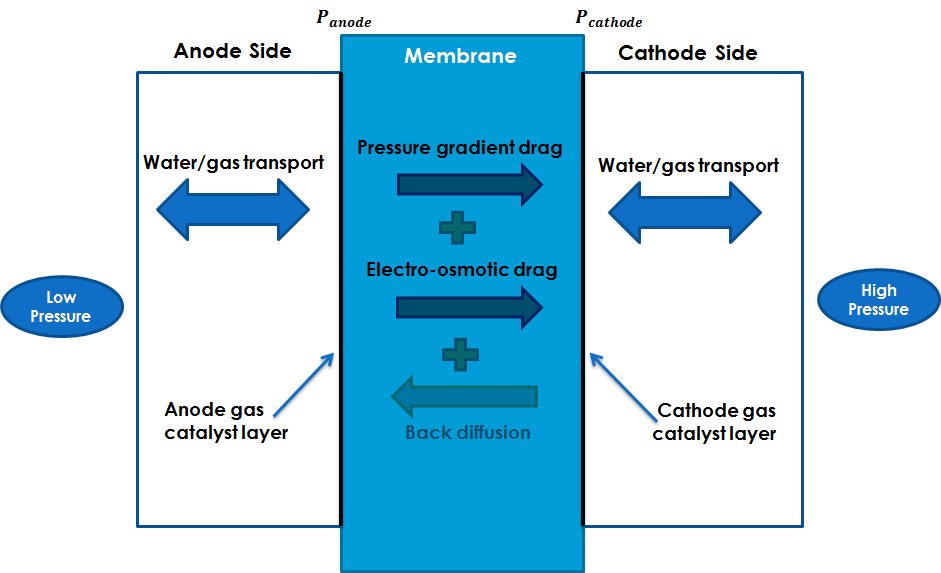
Electrochemical purifier/compressor
Eco-SESA Cross Disciplinary Program (CDP)



