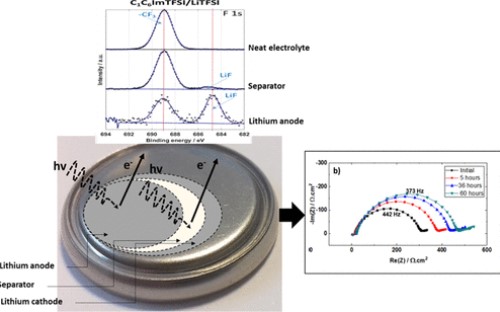
Lithium reactivity toward an electrolytic media and dendrite growth phenomenon constitutes the main drawback for its use as an anode material for the lithium battery technology. Ionic liquids (ILs) were pointed out as promising electrolyte solvent candidates to prevent thermal runaway in a lithium battery system. However, the reactivity of lithium toward such a kind of an electrolyte is still under debate. In this study, the interaction between lithium metal and imidazolium-based ILs, 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (C(1)C(6)ImTFSI) and 1-hexyl-3-methylimidazolium bis(fluorosulfonyl)imide (C(1)C(6)ImFSI), has been investigated based on the nondestructive methodology coupling electrochemical impedance spectroscopy (EIS) and X-ray photoelectron spectroscopy (XPS) in coin cells aged several days at open-circuit voltage. The main components detected by XPS in the bulk separator and at the surface of the lithium metal are the byproducts of cation and anion degradation. Similarities and differences were noticed depending on the anion nature of bis(trifluoromethylsulfonyl)imide versus bis(fluorosulfonyl)imide. The role of lithium salt addition (LiTFSI) was also pointed, giving rise to the stability improvement of the electrolytic solution toward the lithium anode. A direct correlation between the resistance of the bulk electrolyte and of the interface electrolyte/lithium and chemical composition changes were established based on a detailed EIS and XPS combined study.
https://doi.org/10.1021/acsami.9b00753


