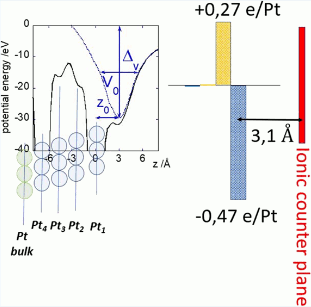
Unraveling the Charge Distribution at the Metal-Electrolyte Interface Coupling in Situ Surface Resonant X-Ray Diffraction with Ab Initio Calculations
par Yvonne Soldo-Olivier, Eric Sibert, Maurizio De Santis, Yves Joly et Yvonne Gründer