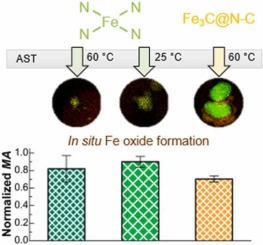
Electrochemical transformation of Fe-N-C catalysts into iron oxides in alkaline medium and its impact on the oxygen reduction reaction activity
by Ricardo Sgarbi, Kavita Kumara, Laetitia Dubau, Vincent Martin, Michel Mermoux & Frédéric Maillard for LEPMI