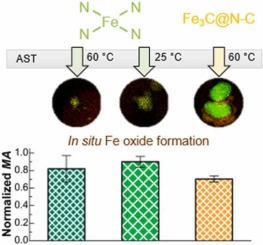
Electrochemical transformation of Fe-N-C catalysts into iron oxides in alkaline medium and its impact on the oxygen reduction reaction activity
par Ricardo Sgarbi, Kavita Kumara, Laetitia Dubau, Vincent Martin, Michel Mermoux et Frédéric Maillard pour le LEPMI