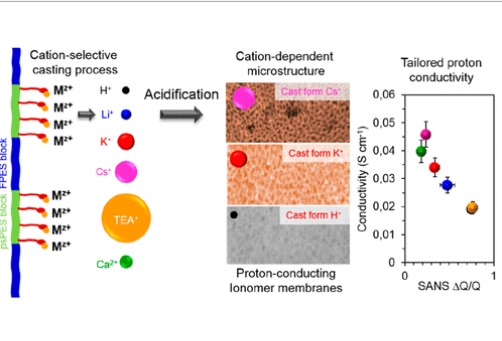
Tailoring the Proton Conductivity and Microstructure of Block Copolymers by Countercation-Selective Membrane Fabrication
Huu-Dat Nguyen, Thi Khanh Ly Nguyen, Emilie Planes, Jacques Jestin, Lionel Porcar, Sandrine Lyonnard, Cristina Iojoiu