Résumé :
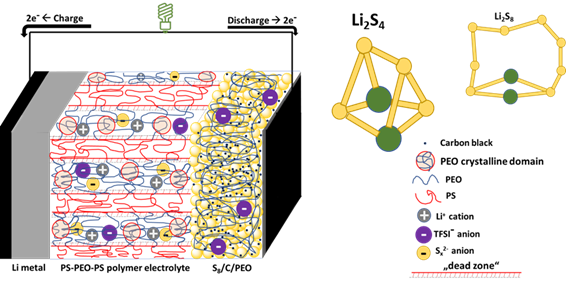
Lithium-sulfur (Li-S) batteries make use of the readily available, non-toxic, high-capacity sulfur positive electrode and Li metal negative electrode to theoretically deliver energy densities up to ten times (2500 Wh/kg) higher than what the current Li-ion battery offers. However, this promising technology faces impediments; the most important is that the intermediate products (lithium polysulfides, Li2Sx, 2 ≤ x ≤ 8) produced in the positive electrode, dissolve and migrate through the electrolyte to the negative electrode in a process referred to as “redox shuttle effect”. At the negative electrode, these Li2Sx react with the Li metal to form an insulating layer which blocks part of the electrochemically active surface area, as well as increases the internal resistance of the cell thereby affecting tremendously its coulombic efficiency and capacity. The “Li2Sx shuttle” may be mitigated using a functionalized solid polymer electrolyte (SPE) by capitalizing on the mechanical properties of nanostructured triblock copolymer polystyrene-poly(ethylene oxide)-polystyrene (SEOS) as well as other functionalities of PEO-based single-ion conducting SPEs. As the Li2Sx are ionic and their “shuttling” behaviour is dependent on the nature of the electrolyte, it is of utmost interest to investigate the interactions between the Li2Sx and these SPEs.
In this thesis, the shuttling of polysulfides in Li-S batteries with polymer electrolytes was investigated. The ionic transport of lithium polysulfides within several SPEs (binary vs. single-ion conducting) has been characterized by differential scanning calorimetry (DSC) to have access to thermodynamic properties such as glass transition (Tg) or melting temperature (Tm) which are linked to the ionic transport properties namely the (i) ionic conductivity, σ, (ii) transference number, T+, and (iii) ambipolar diffusion coefficient, Damb of the ions. These characterizations allow us to gain insight into the redox reactions that take place in Li-S batteries to mimic their failure mechanism. Interestingly, for the binary SPEs (PEO and SEOS) blended with Li2S4, the T+ tends to increase by at least a factor of 4 times higher than the reference PEO/LiTFSI electrolyte. In contrast, for the single-ion SPEs, the presence of lithium polysulfides is weakly felt.
Additionally, the effect of Li2S4 doping into SPEs on the interfacial resistance of the Li metal was also investigated. The results show that, unlike LiTFSI salt, Li2S4 addition is beneficial to the Li/SPE interface as it drastically reduces the interfacial resistance to build a stable interface 10 hours after the cell assembly. To gain more insight into the morphology of the interface, lab-based X-ray tomography has been used to characterize the wetting of the SPE on the Li metal.